Lecture_11_F11
advertisement

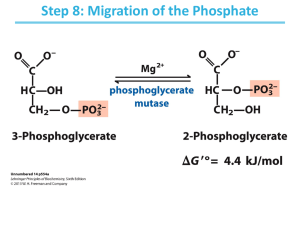
Biochemistry Lecture 11 Gluconeogenesis Gluconeogenesis -Metabolic Pathways are Irreversible ∆G between the 1st & last metabolite is large & neg. - If 2 metabolites are interconvertible (metab 1 metab 2), the path from Metab 1 Metab 2 must be different from that of Metab 2 Metab 1 A B Metab1 Metab2 Y X A. Circumventing Pyruvate Kinase - Conversion of Pyruvate to Phosphoenolpyruvate 1. Carboxylation of pyruvate to oxaloacetate √ 2. Transport of oxaloacetate out of mitochondria Oxaloacetate NADH + H+ NAD+ Malate cyto mito Oxaloacetate NADH + H+ NAD+ Malate Inner mito. Memb. 3. PEP Carboxykinase: decarboxylates and adds phosphate B. Circumventing PFK – dephosphorylation of F1,6BP • Dephosphorylation is not phosphorylation in reverse! • Reverse Phosphorylation of ADP by F1,6 BP to generate F6P (and ATP) would be steeply uphill: F1,6 BP + ADP F6P + ATP ∆G° = +3.4 kcal/mol • Instead, dephosphorylation is carried out: F1,6 BP + H2O F6P + PO4 ∆G° = -3.9 kcal/mol • Reverse Phosphorylation would be mediated by PFK • Dephosphorylation is mediated by F1,6BPase C. Circumventing Hexokinase – dephosphorylation of G6P • Mediated by G6Pase • G6Pase is present only in liver and kidney • Hence, these are the only tissues that can synthesize and secrete glucose into the blood The Gluconeogenic Response is Activated Largely by the State of Feeding/Fasting Glycogen Blood Glucose Blood Glucose Glucose Blood Glucose Pyruvate Alanine Acetyl CoA FA’s The Cory Cycle Gluconeogenesis & Glycolysis can Occur at the Same Time in Different Organs IN MUSCLE IN LIVER Glucose Gluconeogen. Pyruvate B L O O D Glucose Glycolysis Pyruvate Alanine Alanine Lactate Lactate RED BLOOD CELLS Glucose *** The main substrate here is LACTATE*** Pyruvate Lactate Regulation of Metabolism Overview of Energy Metabolism FATS POLYSACCHARIDES PROTEINS Stage I Digestion Fatty Acids, Glucose and Glycerol other sugars Amino Acids Stage II Anaerobic Acetyl CoA ATP ADP O2 eOxidative Phosphorylation TCA cycle CoA CO2 Stage III Aerobic Principles of Regulation • The flow of metabolites through the pathways is regulated to maintain homeostasis • Sometimes, the levels of required metabolites must be altered very rapidly – Need to increase the capacity of glycolysis during the action – Need to reduce the capacity of glycolysis after the action – Need to increases the capacity of gluconeogenesis after successful action Rates of a Biochemical Reaction • Rates of a biochemical reaction depend on many factors • Concentration of reactants • Activity of the catalyst – Concentration of the enzyme – Intrinsic activity of the enzyme • Concentrations of effectors – Allosteric regulators – Competing substrates – pH, ionic environment • Temperature Reactions Far From Equilibrium are Common Points of Regulation Hexokinase • Isozymes are different enzymes that catalyze the same reaction • They typically share similar sequences • Their regulation is often different eg. G6P is structurally similar to glucose, and competes with glucose for active site of hexokinase P PFK Allosteric site Active site Fructose-2,6-bisphosphate Two Alternative Fates for Pyruvate • Pyruvate can be a source of new glucose – Store energy as glycogen – Generate NADPH via pentose phosphate pathway • Pyruvate can be a source of acetyl-CoA – Store energy as body fat – Make ATP via citric acid cycle • Acetyl-CoA stimulates glucose synthesis by activating pyruvate carboxylase Pancreas Adrenal Medulla + Glucagon Liver Epinephrine Brain Muscle Glycogen Glycogen + + + + Glucose (Blood) Glucose F6P + PFK F1,6BP PK Pyruvate F2,6BP Glucose + F6P + PFK F1,6BP PK Pyruvate F2,6BP