Electrophilic Aromatic Substitution: Nitration of Bromobenzene
advertisement
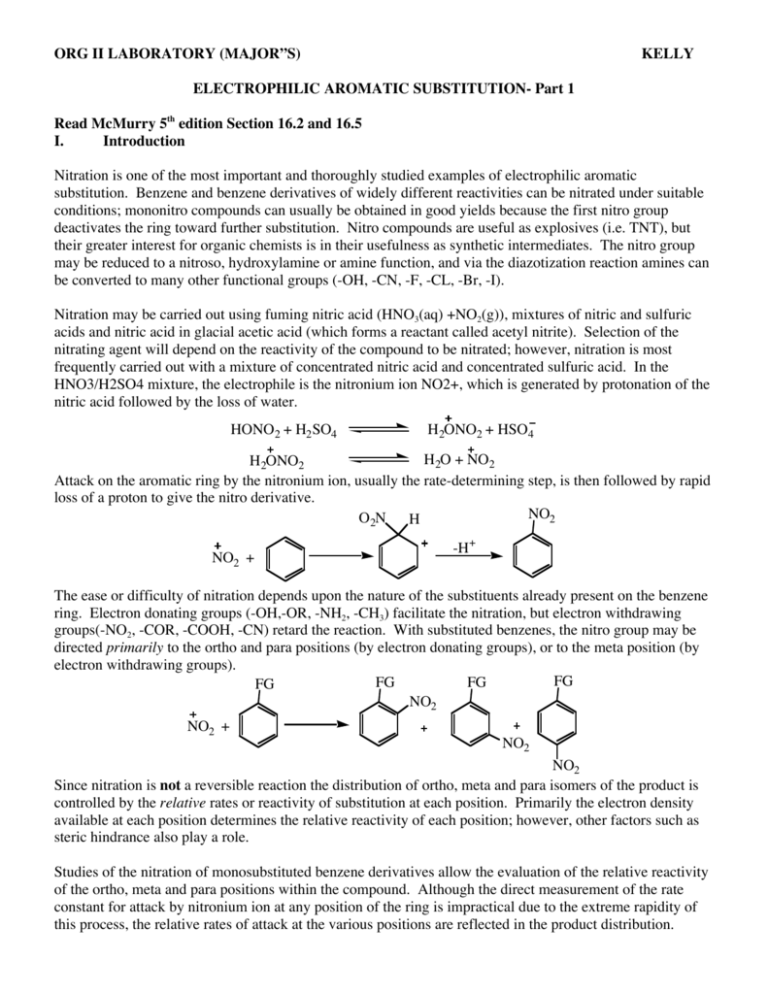
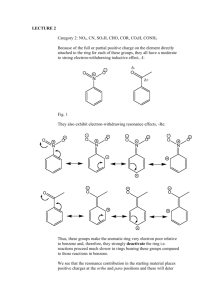
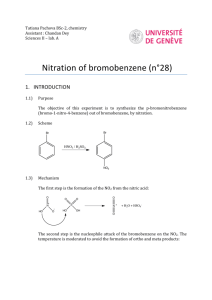
ORG II LABORATORY (MAJOR”S) KELLY ELECTROPHILIC AROMATIC SUBSTITUTION- Part 1 Read McMurry 5th edition Section 16.2 and 16.5 I. Introduction Nitration is one of the most important and thoroughly studied examples of electrophilic aromatic substitution. Benzene and benzene derivatives of widely different reactivities can be nitrated under suitable conditions; mononitro compounds can usually be obtained in good yields because the first nitro group deactivates the ring toward further substitution. Nitro compounds are useful as explosives (i.e. TNT), but their greater interest for organic chemists is in their usefulness as synthetic intermediates. The nitro group may be reduced to a nitroso, hydroxylamine or amine function, and via the diazotization reaction amines can be converted to many other functional groups (-OH, -CN, -F, -CL, -Br, -I). Nitration may be carried out using fuming nitric acid (HNO3(aq) +NO2(g)), mixtures of nitric and sulfuric acids and nitric acid in glacial acetic acid (which forms a reactant called acetyl nitrite). Selection of the nitrating agent will depend on the reactivity of the compound to be nitrated; however, nitration is most frequently carried out with a mixture of concentrated nitric acid and concentrated sulfuric acid. In the HNO3/H2SO4 mixture, the electrophile is the nitronium ion NO2+, which is generated by protonation of the nitric acid followed by the loss of water. HONO2 + H2 SO4 H2ONO2 + HSO4 H2O + NO2 H2ONO2 Attack on the aromatic ring by the nitronium ion, usually the rate-determining step, is then followed by rapid loss of a proton to give the nitro derivative. NO2 O2N H NO2 + -H+ The ease or difficulty of nitration depends upon the nature of the substituents already present on the benzene ring. Electron donating groups (-OH,-OR, -NH2, -CH3) facilitate the nitration, but electron withdrawing groups(-NO2, -COR, -COOH, -CN) retard the reaction. With substituted benzenes, the nitro group may be directed primarily to the ortho and para positions (by electron donating groups), or to the meta position (by electron withdrawing groups). FG FG FG FG NO2 NO2 + NO2 NO2 Since nitration is not a reversible reaction the distribution of ortho, meta and para isomers of the product is controlled by the relative rates or reactivity of substitution at each position. Primarily the electron density available at each position determines the relative reactivity of each position; however, other factors such as steric hindrance also play a role. Studies of the nitration of monosubstituted benzene derivatives allow the evaluation of the relative reactivity of the ortho, meta and para positions within the compound. Although the direct measurement of the rate constant for attack by nitronium ion at any position of the ring is impractical due to the extreme rapidity of this process, the relative rates of attack at the various positions are reflected in the product distribution. Consider the following example of the nitration of benzaldehyde: CHO CHO CHO CHO NO2 NO2 % of nitration product corrected % of nitation product 19% 19/2 (9.5) 72% NO2 9% 72/2 (36) 9/1 (9) RELATIVE REACTIVITY 36/9 9/9 9.5/9 (1.00) (4.00) (1.05) In the example above, there are two ortho positions, two meta positions and one para position where the electrophilic substitution can occur. All other things being equal, we might expect a simple statistical distribution of products (i.e. two of the five possible positions are ortho, or 2/5 = 40% ortho, likewise 2/5 = 40% meta and 1/5 =20% para). This is not the case. To obtain the true relative rates of nitration we must first account for the statistical factors by dividing the % of each position product by the number of positions. By dividing these statistically corrected numbers by the smallest value, the relative reactivities are obtained. It can be seen that the meta position of benzaldehyde is 4 times as reactive as either the ortho or para positions. Thus, the formyl group of benzaldehyde is a meta-directing substituent. II. Nitration of Bromobenzene and Calculation of Relative Reactivities. In this experiment we will carry out the nitration of bromobenzene and determine the relative reactivities of the ortho and para positions in bromobenzene by quantitative analysis of the product mixture using GC analysis. Br Br Br Br NO2 HNO3 H2SO4 NO2 NO2 trace SAFETY NOTE!!!! Concentrated sulfuric and nitric acids can cause severe burns on contact with the skin. Particular care must be taken in measuring and handling these reagents. Clean up any spills immediately (consult with your instructor). Should any acid get on you, wash quickly and thoroughly with cold water. WEAR SAFETY GOGGLES! Aromatic nitro compounds are generally toxic and may cause contact dermatitis, avoid skin contact with any of the reaction products. Diethyl ether is highly flammable. There should be no flames in the room. Procedure In a 125-mL Erlenmeyer flask, place 10 mL of concentrated nitric acid and add carefully 10 mL of concentrated sulfuric acid. Cool the mixture to room temperature (external water bath) and add, in two or three portions 5.5 mL of bromobenzene. Shake the reaction flask continuously and cool it in cold water if necessary to keep the temperature between 50 and 60˚C. After all the bromobenzene has been added and the temperature no longer tends to rise from the heat of the reaction, place the flask in a beaker of boiling water on a hot plate and heat for 1/2 hr with occasional swirling. Cool the flask to room temperature and pour the reaction mixture into about 100 mL of cold water. Suction filter the crude product containing both o- and pnitrobromobenzene. Press on the filter with a cork to help remove water. Dissolve approximately 0.5 g of crude product in 10 mL of diethyl ether. Evaporate the ether to a total volume of about 5 mL and analyze by gas chromatography. Typical column conditions include temp = 170˚C and column pressure at 25-30 psi. The order off the column is typically para (BP-256˚C) then ortho (BP-260C). Dispose of discarded aqueous layers by washing down the drain with ample water. From your gas chromatogram determine the areas under each peak and calculate the percent of ortho and para-nitrobromobenzene in the mixture. Use this data to calculate the relative reactivities of the two positions in bromobenzene. III. Lab Report A. Title Page B. Introduction C. Experimental Description D. Observations and Data 1. Include calculations of relative reactivity of o- and p-positions. E. Conclusion 1. Discuss how the data allows you to determine the directing influence of the bromo group in bromobenzene (i.e. ortho, para director or meta director). 2. Give an explanation as to why the bromo group does as it does.


![[VO(H2O)5]H[PMo12O40]-catalyzed nitration of alkanes with nitric acid](http://s3.studylib.net/store/data/007395962_1-c5684ccdbf5a6a8d13576cb676ea7c0b-300x300.png)