preparation of acetylsalicylic acid (aspirin)
advertisement

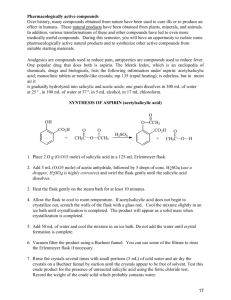
PREPARATION OF ACETYLSALICYLIC ACID (ASPIRIN) BACKGROUND One of the most widely used nonprescription drugs is aspirin. In the United States, more than 15,000 pounds are sold each year. This is not surprising when one considers the medicinal applications for aspirin. It is an effective analgesic (pain killer) that can reduce the mild pain of headache, toothache, neuralgia (never pain), muscle pain, and join pain (from arthritis and rheumatism). Aspirin behaves as an antipyretic drug (it reduces fever) and it is an anti-inflammatory agent capable of reducing swelling and redness associated with inflammation. It is also an effective agent in preventing strokes and heart attacks due to its ability to act as an anticoagulant by preventing platelet aggregation. The father of modern medicine was Hippocrates, who lived sometime between 460 B.C and 377 B.C. Hippocrates left historical records of pain relief treatments, including the use of powder made from the bark and leaves of the willow tree to help heal headaches, pains and fevers. By 1829, scientists discovered that it was the compound salicin in willow plants that gave pain relief. It was a short time later that a tiny amount of bitter tasting, yellow, needle-like crystals were isolated from willow bark by Johann Buchner, professor of pharmacy at the University of Munich. By 1829, a French chemist improved the extraction procedure and was able to obtain about 30g from 1.5kg of bark. In 1838, an Italian chemist was able convert salicin to an acid of crystallized, colorless needles, which he named salicylic acid. Since salicylic acid contains phenolic and carboxylic acid groups, it was irritating to the mouth, throat, and stomach. After considerable work, the derivative acetylsalicylic acid was found to have similar painrelieving properties without the harsh side effects. It was in 1899, that the Bayer Company in Germany patented the ester acetylsalicylic acid and marketed the product as “aspirin” in 1899. Their studies showed that this material was less of an irritant and the acetylsalicylic acid hydrolyzed in the small intestine to salicylic acid, which was then absorbed into the bloodstream. The relationship between salicylic acid and aspirin is shown in Figure 1. COOH COOH OH Salicylic Acid O O C CH3 Acetylsalicylic Acid (Aspirin) Fig. 1: Aspirin vs. Salicylic Acid The first person to do so was a French chemist named Charles Frederic Gerhardt. In 1853, Gerhardt neutralized salicylic acid by buffering it with sodium (sodium salicylate) and acetyl chloride, creating acetylsalicylic acid. Gerhardt's product worked but he had no desire to market it and abandoned his discovery. In 1899, a German chemist named Felix Hoffmann (Fig. 2), who worked for a German company called Bayer, rediscovered Gerhardt's formula. Felix Hoffmann made some of the formula and gave it to his father who was suffering from the pain of arthritis, with good results. Felix Hoffmann then convinced Bayer to market the new wonder drug. Aspirin was patented on February 27, 1900. The folks at Bayer came up with the name Aspirin, it comes from the 'A" in acetyl chloride, the "spir" in spiraea ulmaria (the plant they derived the salicylic acid from) and the 'in' was a then familiar name ending for medicines. Aspirin was first sold as a powder. In 1915, the first Aspirin tablets were made. Interestingly, Aspirin ® was once a trademark belonging to Bayer. After Germany lost World War I, Bayer was forced to give it up as part of the Treaty of Versailles in 1919. Fig. 2: Felix Hoffman Fig. 3: Bayer was the first company to market Aspirin. Fig. 4: Pure Acetyl Salicylic Acid (Aspirin) SYNTHESIS Chemistry O O O OH O OH OH O + + CH3 O Acetic Anhydride O CH3 CH3 O Salicylic Acid Acetyl Salicylic Acid Figure 5: The Aspirin Synthesis Reaction CH3 OH Acetic Acid Safety The chemicals used to synthesize aspirin present a health hazard and exposure should be kept to a minimum: 1) Sulfuric Acid: A highly corrosive liquid that will cause severe burns if it comes into contact with skin. 2) Salicylic Acid: Harmful by inhalation, ingestion, and through skin absorption. An irritant. 3) Acetic anhydride: Poison. Corrosive. Cuases severe burns. Harmful if swallowed or inhaled. Causes severe respiratory irritation. Eye contact may cause serious irritation or burns. 4) Ferric Chloride Solution: Corrosive 5) Ethanol: Toxic and flammable Procedure 1. Set up a secure water bath, as shown in the to the right, using a ring stand, extension (which will be used to hold a 125 mL Erlenmeyer flask), and a hotplate. Begin heating the water. A 600 mL beaker will accommodate the flask well. figure clamp 2. Weigh approximately 2 grams of salicylic using a watch glass, on the top loading balance. Record the weight to the full precision of the balance. acid, 3. Transfer the salicylic acid completely to a dry 125 mL Erlenmeyer flask. clean, 4. In the hood, transfer 4.0 mL of acetic anhydride into the 125 mL Erlenmeyer flask using a 10 mL graduated cylinder. 5. In the hood, carefully add 5 drops of concentrated sulfuric acid to the Erlenmeyer flask using the dropper provided. Be careful not to let the sulfuric acid drop anywhere other than into the flask. If any acid does spill anywhere, advise the teaching assistant immediately. Swirl the flask to help mix the contents. Do not add the sulfuric acid before the acetic anhydride. The reaction mixture should form a clear solution as it is heated. 6. Bring the flask back to the desk. Place it in the extension clamp (tilting the flask will help minimize boil over). Make sure the reaction mixture is below the liquid level in the bath and that the water is boiling. Heat in the boiling water bath for not less than 20 minutes by which time all of the solid should have dissolved. 7. Obtain 50 mL of ice water in a 150 mL beaker. After removing the flask from the water bath, measure 10 mL of ice water in a 10 mL graduated cylinder and add it to the reaction mixture, 33-5 5 drops at a time, to hydrolyze the unreacted acetic anhydride. nmeyer flask in an ice bath with occasional swirling to cause precipitation of the product. 8. Chill the Erlenmeyer Add 25 mL of ice water to the reaction mixture. If no precipitate forms, scratching the side of the flask with a glass stirring rod may help initiate crystallizatio crystallization. n. While the flask is chilling, prepare a vacuum filtration apparatus as shown in the figure to the right. 9. Weigh a piece of filter paper on a watchglass. Record the weight of the paper + the watchglass. Place the weighed paper in the Buchner funnel. Wet et the paper with a small amount of water using your wash bottle to seal the paper to the funnel surface. 10. Turn on the aspirator. Swirl the flask and pour its contents carefully onto the center of the filter paper. Use a stirring rod with a rubber poli policeman to assist in getting all of the solid out of the flask. 11. When all the solid has been captured on the filter paper and the liquid has all been sucked through, wash the product by slowly pouring a measured amount of ice water, not to exceed 15 mL, on the surface of the precipitate. Decrease the aspirator flow and adjust it so the liquid is removed at a very slow drop rate. This is to wash the precipitate to remove any remaining acetic acid. 12. Take a very small amount (50 mg) of the sample and pla place ce it on a watch glass. We will use this amount to perform some tests. It can be dried on the laboratory ventilators. 13. Transfer the filter paper and remaining product back to the watch glass by inserting a clean spatula under the bottom of the filter paper aper to peel it away from the funnel. Weigh the product and weigh paper. Qualitative Tests of the Purity of the Product: 1) Ferric Chloride Test The addition of ferric chloride to salicylic acid produces a specific color caused by the reaction of salicylic ic acid with aqueous ferric (Fe(H2O)6+3) ion. The oxygen atoms of the acid group –COOH, and of the -OH OH group on the salicylic acid together can form a complex with Fe(H2O)6+3. That complex has an intense violet color. In aspirin, the –OH group of salicylic acid has been replaced by a O-COCH3 O group which prevents the second bond from being formed. The resulting complex with aspirin shows only a slight yellow color, not very different from that of Fe(H2O)6 +3 itself. Into each of three clean and dry small ttest est tubes, place 1 mL of ethanol (ethyl alcohol) and one drop of 1% aqueous ferric chloride solution. Into the first, place a few crystals (about 5 mg) of salicylic acid. Into the second, place the same amount of your product from the 50 mg sample on the watch glass in step 12, above. The third tube is used as a control (blank). Shake the mixture in each test tube. Record the results in your notebook and on the data sheet. Comment on the significance of the results – in particular, how it relates to the purity of your product. 2) Heat Test Aspirin is known to be unstable in the presence of moisture at elevated temperatures. At those temperatures, aspirin hydrolyzes (i.e., reacts with water) to decompose into salicylic acid and acetic acid. Acetic acid has a characteristic pungent smell. The detection of this odor depends on the assumption that all of the acetic acid (which was both a product in the reaction and produced from the hydrolysis of excess acetic anhydride) was removed from the sample in the final washing process. To another clean small test tube, add a few crystals (about 10 mg) of your product, again, from the 50 mg sample on the watch glass in step 12, above. Heat it gently to melting. Remove from the heat and note the odor of the escaping vapor by carefully fanning the vapors with your hand toward your nose. Comment on the odor and on the implications on the purity of your product. ACKNOWLEDGEMENTS http://inventors.about.com/library/inventors/blaspirin.htm, “The History of Aspirin” by Mary Bellis, 15 July 2009. http://en.wikipedia.org/wiki/Aspirin#History, “Aspirin”, 15 July 2009. http://en.wikipedia.org/wiki/File:Acetylsalicylicacid-crystals.jpg, “Aspirin in it’s Pure State”, Retouched photo of Thesmallprint189 + Leridant (modification of colour) source: http://en.wikipedia.org/wiki/Image:Ace, 15 July 2009. REPORT SHEET 1. Theoretical Yield _______ 1 1 138 138 1 1 = ___________ g aspirin 2. Actual Yield a) Mass of empty 50mL beaker ___________ g b) Mass of aspirin + beaker ___________ g c) Mass of aspirin (b – a) ___________ g d) Percent yield [(c/1) x 100% ___________ % 3. Iron (III) Chloride Test SAMPLE a) Salicylic acid b) Your aspirin c) Commercial aspirin COLOR INTENSITY 4. Post-lab Questions a) What was used to increase the rate of the reaction? b) What would happen to your percent yield if the aspirin you prepared was not dried completely? c) A student’s theoretical yield was 12.0g of aspirin, but he only obtained 7.5g. What was his percent yield? d) Did you like this experiment? (Please explain why or why not.)