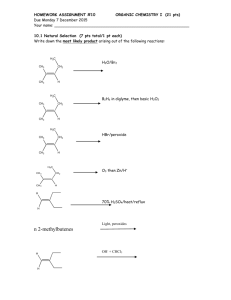
Chem 233: Problem Set #1 (on Chapter 1)

1.
2.
3.
4.
5.
Chem 233: Problem Set #1 (on Chapter 1)
What type of hybridization is used by each non-hydrogen atom in the following substances?
O
CH
3
HC C
HC
CH
3 H
2
C
H
3
C C O
C
H
3
C CH H
CH
3 CH
3
HC CH
2
SH
O H
C C N
HC C
C CH
3
HO N
HC CH
CH
3
C
H
Draw Lewis (or Kekule) structures for all substances shown below. For those substances in E through H, draw all possible constitutional isomers.
A. HNO
B.
C.
H
2
BF
SO
3
D. PH
3
2
3
E.
F.
C
4
H
10
C
4
H
8
G. C
4
H
6
H. C
4
H
8
Cl
2
I. NO
2
+
+
J. C
2
H
5
K. NH
2
-
L. HSO
3
+
M. CS
2
N. CO
3
-2
Predict the molecular shape and bond angles for each substance given in question #2.
Draw a Lewis structure for CH
3
CN and identify what type of hybridization each atom uses during bonding. Use valence bond theory to draw orbital pictures showing the orbitals or hybrids involved in covalent bond formation in CH
3
CN.
Consider the singly occupied orbitals shown below. Decide whether each set of orbitals, when overlapped along the specified direction, will lead to formation of a F -type bond, a
B -type bond, or neither type bond
6.
7.
For each line (skeletal) structure shown below, write a structural formula that includes all hydrogen atoms.
O
I.
II.
III.
O
H
IV.
VII.
X.
O
O
OH
NH
2
V.
O
VIII.
Br
Br
N
VI.
IX.
O
O
Draw the following structural formulas as line (skeletal) structures.
A.
H
3
C H
H
3
C
CH
CH
3
2
C C
H
C CH
3
B.
H
3
C
H
3
C
CH
3
CH
3
C
H
C CH CH CH
3
C.
CH
2
CH
3
H
3
C
HC
H
2
C
CH
HC CH
C
H
H
2
C CH
CH
3
CH
3
D.
O
H
3
C C C CH
2
C OH
OH
8. Calculate formal charges for each atom indicated with an arrow.
H
3
C
HC
C
H
C
C
C
H
Cl
H
3
C
H
2
C
O
CH
3
C
H
CH
3
HC
HC
H
C
N
CH
3
O
CH
CH
HC
C
H
CH
H
3
C
CH
3
C
H
3
C
CH
3
C
CH
3
H
3
C C
H
CH
3
OH
2
9. Draw all possible resonance structures for nitrite, NO
2
-
. Calculate the formal charges on all atoms in each resonance structure. Show with curved arrows how one resonance structure is converted to the other by movement of electron pairs. Draw a picture of the resonance hybrid and indicate the N-O bond orders in this hybrid.
10. Draw all possible resonance structures for each of the following. Indicate whether each resonance structure is of equivalent energy to the original.
A.
H
3
C
NH
C
H
C
NH
C CH
3
B.
Cl
H
C.
O
D.
E.
N
F.
H
3
C
O
C
H
O
CH
3