The Determination of Vitamin C
advertisement

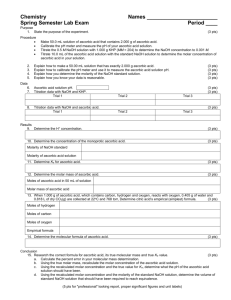
Exercise 5 Illinois Central College CHEMISTRY 130 Laboratory Section:__________ Page 1 Name:_________________________ Chemical Stoichiometry: The Determination of Vitamin C Objectives This experiment will illustrate the stoichiometric relationships between reactants in a chemical process. This stoichiometric relationship represents the mole:mole ratios of reactants and products to one another in a balanced chemical reaction. This experiment also uses the concept of Molarity, which represents the concentration of a solution in moles of solute per liter of solution and allows the chemist to relate the volume of a given solution to the number of moles of dissolved solute present. That is, moles solute = (volume of solution used, L) x (Molarity of the solution) We will also address the concept of the percent purity of an unknown. This is calculated by dividing the amount of pure substance found in a sample by the actual sample mass and multiplying this quotient by 100. Background Vitamins are a group of organic substances required in the diet of man and animals. Vitamin C, or ascorbic acid, was first isolated as a pure substance by Albert Azent-Gyorgi and Charles King in 1928. It can be obtained from citrus fruits, tomatoes, potatoes, and fresh vegetables, particularly from red and green peppers. It is now made synthetically. All animals except man, other primates, and guinea pigs, are capable of synthesizing their own ascorbic acid; man must obtain it from food. The long-established use of citrus fruits (particularly limes) to prevent the occurrence of shipboard scurvy found ready explanation when it was established that the condition is connected with a deficiency of Vitamin C. This anti-scorbutic use is the origin of the common name, ascorbic acid. Well-balanced diets provide adequate amounts of the vitamin as measured by the Recommended Daily Allowance (RDA) of about 75 mg per day. The use of large, regular, supplementary doses of Vitamin C (megavitamin therapy) in prophylaxis and therapy of the common cold and other medical conditions has been strongly advocated, particularly by Linus Pauling. Such use is the subject of lively controversy with the final verdict not yet in sight. Ascorbic acid is a cheap, comparatively simple, water-soluble, organic compound having the molecular formula, HC6H706 . It is a weak mono-protic acid and may be titrated with a known concentration of sodium hydroxide solution, NaOH(aq), to a phenolphthalein end-point in order to determine the amount of Vitamin C present. It oxidizes readily in air, particularly at higher temperatures, and hence the the vitamin is easily destroyed in cooking and in long storage. The oxidation of ascorbic acid by iodate salts is rapid and quantitative, that is, if we monitor the amount of iodate used to oxidize a sample containing vitamin C, we can determine the exact amount in the sample. Exercise 5 Page 2 We shall use a procedure in which a solution sample of ascorbic acid is first titrated with NaOH (sodium hydroxide) and then, after reacidification, with potassium iodate. The two relevant equations are: HC6H7O6(aq) + NaOH(aq) 3 HC6H7O6(aq) + KIO3(aq) NaC6H7O6(aq) + H2O 3 C6H6O6(aq) + KI(aq) + 3 H2O Note that the mole:mole relationship of ascorbic acid to sodium hydroxide is 1:1, meaning one mole of ascorbic acid reacts with one mole of base. On the other hand the mole:mole relationship of ascorbic acid to potassium iodate is 3:1, so that three moles of ascorbic acid are oxidized by one mole of potassium iodate. Phenolphthalein serves as an indicator in the titration with sodium hydroxide. When we have added sufficient NaOH to neutralize the ascorbic acid in our sample, the phenolphthalein will change from clear to pink indicating the endpoint of the reaction. The reaction with potassium iodate titration utilizes starch as the indicator. When all of the ascorbic acid has reacted with the KIO3, the excess KIO3 oxidizes the KI produced in the reaction (which is colorless) to I2 which forms a deep blue color with the starch indicating that the reaction is complete. Procedure A. Prepare a solution of 10 mL of 1 M HCl and 1 mL of starch solution in a 50 mL beaker and set it aside. Accurately weigh out a sample of ascorbic acid (about 0.250 g) and place it in a 250 mL Erlenmeyer flask. Add about 50 mL of distilled water and swirl the flask until the mixture is homogeneous. Add 4 drops of phenolphthalein indicator to the Erlenmeyer flask and titrate rapidly, while swirling the flask, with standard (0.100 M) NaOH to the pink endpoint. (As you approach the endpoint, the pink color will begin to persist. At that point, begin to add the titrant drop by drop such that the last drop added causes a persistent pink color that will not swirl away.) Quickly note your titer (the amount of solution used from your Swirl the liquid during the titration burette) then reacidify the sample by adding the HCl/starch and wash the walls mixture you previously prepared. (The former will convert the with distilled water from the wash bottle. ascorbate ion back to unionized ascorbic acid. The latter will act as the endpoint indicator in the second titration.) Speed is of the essence, since solutions of ascorbic acid oxidize rapidly in the presence of sodium hydroxide. Titrate with the standard (0.0300 M) KI03 solution until the first appearance of a blue endpoint. (If the starch is omitted, the endpoint will be signaled, less accurately, by the appearance of a yellow color.) Repeat entire procedure with a second sample. B. Obtain a Vitamin C tablet from the dispensing table. Weigh the tablet, then crush it using a mortar and pestle and rinse all of the resulting powder (using distilled water) into a 250 mL Erlenmeyer flask. Carry out the procedure outlined in Part A using the crushed tablet instead of the ascorbic acid sample. Repeat the entire procedure with a second tablet of the same unknown. Exercise 5 Illinois Central College CHEMISTRY 130 Laboratory Section:__________ Name:_________________________ REPORT SHEET Chemical Stoichiometry: The determination of Vitamin C A.Determination of Purity of Ascorbic Acid Titration with NaOH (0.100 M) Trial 1 Trial 2 Trial 1 Trial 2 Mass of ascorbic acid Initial buret reading Final buret reading Volume of NaOH used Moles of NaOH used Moles of ascorbic acid neutralized Grams of ascorbic acid Percent purity of ascorbic acid Average purity of ascorbic acid Titration with KIO3 (0.0300 M) Initial buret reading Final buret reading Volume of KIO3 used Moles of KIO3 used Moles of ascorbic acid reacted Grams of ascorbic acid Percent purity of ascorbic acid Average purity of ascorbic acid Page 3 Exercise 5 Page 4 B. Determination of Ascorbic Acid in Vitamin C Tablets Titration with NaOH (0.100 M) Trial 1 Trial 2 Trial 1 Trial 2 Mass of tablet Initial buret reading Final buret reading Volume of NaOH used Moles of NaOH used Moles of ascorbic acid neutralized Grams of ascorbic acid Milligrams of ascorbic acid per tablet Percent ascorbic acid in tablet Average percent ascorbic acid Titration with KIO3 (0.0300 M) Initial buret reading Final buret reading Volume of KIO3 used Moles of KIO3 used Moles of ascorbic acid neutralized Grams of ascorbic acid Milligrams of ascorbic acid per tablet Percent ascorbic acid in tablet Average percent ascorbic acid On a separate sheet, show set-ups and calculations for Trial 1 for both the powder and tablet. Exercise 5 Illinois Central College CHEMISTRY 130 Laboratory Section:__________ PRELAB: Exp. 5 Page 5 Name:_________________________ Chemical Stoichiometry: The Determination of Vitamin C. SHOW YOUR WORK 1. A 0.258 g sample of a powder containing some ascorbic acid required 14.50 mL of 0.100 M NaOH for complete neutralization. Calculate the purity of the ascorbic acid. moles of NaOH used in the titration __________________ moles of ascorbic acid reacted __________________ grams of ascorbic acid in sample __________________ Percent purity of the powder __________________ 2. A tablet of Vitamin C weighed 0.306 g and required 15.70 mL of 0.0300 M KIO3 solution for oxidation to the blue starch-iodine endpoint. Calculate the percent ascorbic acid in the tablet. moles of KIO3 used in the titration _________________ moles of ascorbic acid reacted _________________ grams of ascorbic acid in tablet _________________ Percent ascorbic acid in tablet _________________ Exercise 5 Page 6