Crystalline Solid Structures: Atomic Arrangement & Density
advertisement

제3장: 결정질 고체의 구조
ISSUES TO ADDRESS...
• How do atoms assemble into solid structures?
• How does the density of a material depend on
its structure?
• When do material properties vary with the
sample (i.e., part) orientation?
Chapter 3 - 1
학습목표...
•
원자/분자 구조에서 결정질과 비결정질 재료의 차이점 분석
•
면심입방격자, 체심입방격자, 육방조밀격자의 제도
•
면심입방격자와 체심입방격자 구조에서 단위정 모서리 길이와 원자
반지름 사이의 관계식 유도
•
주어진 단위정 차원에서 면심입방격자와 체심입방격자 구조를 갖는
금속의 밀도 계산
•
세 방향 지수가 주어질 때, 단위정 내에 이 지수와 일치하는 방향 제도
•
단위정 내에 그려진 면의 Miller 지수 표기
•
원자의 조밀 충진면으 적층에 의해 면심입방격자와 육방조밀격자가
생기는 과정을 설명
•
단결정과 다결정 재료의 구분
•
재료 성질에서 등방성과 이방성의 차이
Chapter 3 - 2
Energy and Packing
• 치밀않음, 불규칙배열(random packing)
Energy
typical neighbor
bond length
typical neighbor
bond energy
• 치밀, 규칙배열(ordered packing)
r
Energy
typical neighbor
bond length
typical neighbor
bond energy
r
치밀하고 규칙적인 배열의 구조는 낮은 에너지상태를 갖는
경향이 있다.
Chapter 3 - 3
Materials and Packing
결정질 재료(Crystalline materials...)
• 장범위 규칙적 원자배열, 3차원적 패턴
• 예:
-모든 금속
-대부분의 세라믹
-일부의 폴리머
crystalline SiO2
Adapted from Fig. 3.23(a),
Callister & Rethwisch 8e.
Si
비결정질 재료(Noncrystalline materials...)
• 장범위 원자의 규칙성 없음
• 예:
-복잡한 구조
-급냉(rapid cooling)
“Amorphous" = Noncrystalline
Oxygen
noncrystalline SiO2
Adapted from Fig. 3.23(b),
Callister & Rethwisch 8e.
Chapter 3 - 4
금속의 결정 구조
• How can we stack metal atoms to minimize
empty space?
2-dimensions
vs.
Now stack these 2-D layers to make 3-D structures
Chapter 3 - 5
금속의 결정 구조
• 조밀한 원자 충진
• 이유:
- 일반적으로 오직 한 성분만이 존재, 같은 크기의 원자
- 금속결합: 방향성 무
- 작은 최인접 원자간 거리: 낮은 결합에너지
- 개개의 전자 구름은 중심부를 보호하고 있음(Electron cloud
shields cores from each other)
• 가장 간단한 결정구조
We will examine three such structures...
Chapter 3 - 6
단순입방정(Simple Cubic Structure (SC))
• 낮은 충진밀도: 흔하지 않음 (only Polonium(Po) has this structure)
• 입방정의 모서리: 조밀방향 (Close-packed directions)
• 배위수(Coordination #) = 6
[최인접원자수(# nearest neighbors)]
(Courtesy P.M. Anderson)
http://bcs.wiley.com/hebcs/Books?action=mininav&bcsId=5242&itemId=0470419970&assetId=19905
3&resourceId=19134
Chapter 3 - 7
원자충진율(Atomic Packing Factor (APF))
APF =
Volume of atoms in unit cell*
Volume of unit cell
*assume hard spheres
• 단순입방정의 APF = 0.52
atoms
unit cell
a
R=0.5a
APF =
volume
atom
4
p (0.5a) 3
1
3
a3
close-packed directions
contains 8 x 1/8 =
1 atom/unit cell
Adapted from Fig. 3.24,
Callister & Rethwisch 8e.
volume
unit cell
Chapter 3 - 8
체심입방정[Body Centered Cubic Structure (BCC)]
• 체심과 모서리 원자는 입방의 대각선상에 서로 접촉
--Note: 모든 원자는 동일; 중심부의 다른 색깔의 원자는 쉽게 구별하기 위함
ex: Cr, W, Fe (), Tantalum, Molybdenum
• 배위수(Coordination #) = 8
Adapted from Fig. 3.2,
Callister & Rethwisch 8e.
(Courtesy P.M. Anderson)
2 atoms/unit cell: 1 center + 8 corners x 1/8
Chapter 3 - 9
원자충진율(Atomic Packing Factor: BCC)
• APF for a body-centered cubic structure = 0.68
3a
a
2a
Adapted from
Fig. 3.2(a), Callister &
Rethwisch 8e.
R
a
Close-packed directions:
length = 4R = 3 a
atoms
volume
4
p ( 3a/4 ) 3
2
unit cell
atom
3
APF =
volume
3
a
unit cell
Chapter 3 - 10
면심입방정[Face Centered Cubic Structure (FCC)]
• 원자는 입방의 모서리와 면의 중심에 위치
--Note: All atoms are identical; the face-centered atoms are shaded
differently only for ease of viewing.
ex: Al, Cu, Au, Pb, Ni, Pt, Ag
• Coordination # = 12
Adapted from Fig. 3.1, Callister & Rethwisch 8e.
(Courtesy P.M. Anderson)
4 atoms/unit cell: 6 face x 1/2 + 8 corners x 1/8
Chapter 3 - 11
원자충진율(Atomic Packing Factor: FCC)
• APF for a face-centered cubic structure = 0.74
최대의 APF
Close-packed directions:
length = 4R = 2 a
2a
a
Adapted from
Fig. 3.1(a),
Callister &
Rethwisch 8e.
Unit cell contains:
6 x 1/2 + 8 x 1/8
= 4 atoms/unit cell
atoms
volume
4
3
p ( 2a/4 )
4
unit cell
atom
3
APF =
volume
3
a
unit cell
Chapter 3 - 12
FCC 적층 배열
• ABCABC... Stacking Sequence
• 2D Projection
B
B
C
A
B
B
B
A sites
C
C
B sites
B
B
C sites
• FCC Unit Cell
A
B
C
Chapter 3 - 13
조밀육방정[Hexagonal Close-Packed Structure (HCP)]
• ABAB... Stacking Sequence
• 3D Projection
c
a
• 2D Projection
A sites
Top layer
B sites
Middle layer
A sites
Bottom layer
Adapted from Fig. 3.3(a),
Callister & Rethwisch 8e.
• Coordination # = 12
• APF = 0.74
• c/a = 1.633
6 atoms/unit cell
ex: Cd, Mg, Ti, Zn
Chapter 3 - 14
이론밀도(Theoretical Density, r)
Density = r =
r =
where
Mass of Atoms in Unit Cell
Total Volume of Unit Cell
nA
VC NA
n = number of atoms/unit cell
A = atomic weight
VC = Volume of unit cell = a3 for cubic
NA = Avogadro’s number
= 6.022 x 1023 atoms/mol
Chapter 3 - 15
Theoretical Density, r
r =
• Ex: Cr (BCC)
A = 52.00 g/mol
R = 0.125 nm
n = 2 atoms/unit cell
Adapted from
Fig. 3.2(a), Callister &
Rethwisch 8e.
atoms
unit cell
r=
volume
unit cell
R
a
2 52.00
a3 6.022 x 1023
nA
VC NA
a = 4R/ 3 = 0.2887 nm
g
mol
rtheoretical = 7.18 g/cm3
ractual
atoms
mol
= 7.19 g/cm3
Chapter 3 - 16
재료의 밀도
일반적으로
rmetals > rceramics > rpolymers
30
왜?
금속(Metals have...)
Ceramics have...
• less dense packing
• often lighter elements
Polymers have...
r (g/cm3 )
• 조밀충진(close-packing)
(metallic bonding)
• 대부분 큰 원자질량
• low packing density
(often amorphous)
• lighter elements (C,H,O)
Composites have...
• intermediate values
Metals/
Alloys
20
Platinum
Gold, W
Tantalum
10
Silver, Mo
Cu,Ni
Steels
Tin, Zinc
5
4
3
2
1
0.5
0.4
0.3
Titanium
Aluminum
Magnesium
Graphite/
Ceramics/
Semicond
Composites/
fibers
Polymers
B ased on data in Table B1, Callister
*GFRE, CFRE, & AFRE are Glass,
Carbon, & Aramid Fiber-Reinforced
Epoxy composites (values based on
60% volume fraction of aligned fibers
in an epoxy matrix).
Zirconia
Al oxide
Diamond
Si nitride
Glass -soda
Concrete
Silicon
G raphite
PTFE
Silicone
PVC
PET
PC
HDPE, PS
PP, LDPE
Glass fibers
GFRE*
Carbon fibers
CFRE *
A ramid fibers
AFRE *
Wood
Data from Table B.1, Callister & Rethwisch, 8e.
Chapter 3 - 17
Crystals as Building Blocks
• Some engineering applications require single crystals:
-- diamond single
crystals for abrasives
(Courtesy Martin Deakins,
GE Superabrasives,
Worthington, OH. Used with
permission.)
-- turbine blades
Fig. 8.33(c), Callister &
Rethwisch 8e. (Fig. 8.33(c)
courtesy of Pratt and
Whitney).
• Properties of crystalline materials
often related to crystal structure.
-- Ex: Quartz fractures more easily
along some crystal planes than
others.
(Courtesy P.M. Anderson)
Chapter 3 - 18
Polycrystals
이방성(Anisotropic)
• Most engineering materials are polycrystals.
Adapted from Fig. K,
color inset pages of
Callister 5e.
(Fig. K is courtesy of
Paul E. Danielson,
Teledyne Wah Chang
Albany)
1 mm
<Nb-Hf-W plate with an electron beam weld> 등방성(Isotropic)
• Each "grain" is a single crystal.
• If grains are randomly oriented,
overall component properties are not directional.
• Grain sizes typically range from 1 nm to 2 cm
(i.e., from a few to millions of atomic layers).
Chapter 3 - 19
단결정(Single) vs 다결정(Polycrystals)
• Single Crystals
E (diagonal) = 273 GPa
Data from Table 3.3,
Callister & Rethwisch
8e. (Source of data is
R.W. Hertzberg,
Deformation and
Fracture Mechanics of
Engineering Materials,
3rd ed., John Wiley and
Sons, 1989.)
-Properties vary with
direction: anisotropic.
-Example: the modulus
of elasticity (E) in BCC iron:
• Polycrystals
-Properties may/may not
vary with direction.
-If grains are randomly
oriented: isotropic.
(Epoly iron = 210 GPa)
-If grains are textured,
anisotropic.
E (edge) = 125 GPa
200 mm
Adapted from Fig.
4.14(b), Callister &
Rethwisch 8e.
(Fig. 4.14(b) is courtesy
of L.C. Smith and C.
Brady, the National
Bureau of Standards,
Washington, DC [now
the National Institute of
Standards and
Technology,
Gaithersburg, MD].)
Chapter 3 - 20
동질이상(Polymorphism)
• 하나 이상의 결정구조를 갖는 재료[Two or more distinct
crystal structures for the same material (allotropy/polymorphism)]
titanium
, -Ti
carbon
diamond, graphite
iron system
liquid
BCC
1538ºC
-Fe
FCC
1394ºC
-Fe
912ºC
BCC
-Fe
Chapter 3 - 21
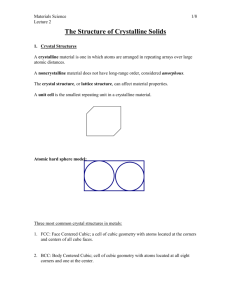
결정계(Crystal Systems)
단위정(Unit cell): 결정의 완벽한 격자 형태를
포함하는 가장 작은 반복 체적(smallest repetitive volume
which contains the complete lattice pattern of a crystal)
7 crystal systems
14 crystal lattices
a, b, and c are the lattice constants
Fig. 3.4, Callister & Rethwisch 8e.
Chapter 3 - 22
입방
육방
정방
삼방
사방
단사
삼사
Chapter 3 - 23
점 좌표(Point Coordinates)
z
단위정 중심에 대한 점 좌표(Point
coordinates for unit cell center are)
111
c
a/2, b/2, c/2
y
000
a
x
½ ½ ½
b
단위정 꼭지점에 대한 점 좌표(Point
coordinates for unit cell corner are)
111
z
2c
b
b
y
평행이동(Translation): 격자상수를
곱하여 정수로 표시(integer multiple
of lattice constants) 결정격자
내에서는 동일한 위치(identical
position in another unit cell)
Chapter 3 - 24
결정 방향(Crystallographic Directions)
z
방법(Algorithm)
1. 적절한 크기의 벡터를 좌표축의 중심을
지나도록 한다.
2. 벡터가 각 축에 투영되는 길이를 결정하여
단위정의 크기인 a, b, c로 나타낸다.
y 3. 최소의 정수로 표기한다.
4. 콤마로 분리하지 않고, 모난 괄호 안에
표시한다.
x
[uvw]
ex: 1, 0, ½
=> 2, 0, 1 => [ 201 ]
-1, 1, 1 => [ 111 ]
위의 바(overbar)는 음의
지수(음의 좌표)를 나타냄
동등한 방향들을 묶어 족으로 표시(families of directions) <uvw>
Chapter 3 - 25
선밀도(Linear Density)
• Linear Density of Atoms LD =
Number of atoms
Unit length of direction vector
[110]
ex: linear density of Al in [110]
direction
a = 0.405 nm
# atoms
a
Adapted from
Fig. 3.1(a),
Callister &
Rethwisch 8e.
LD =
length
2
= 3.5 nm-1
2a
Chapter 3 - 26
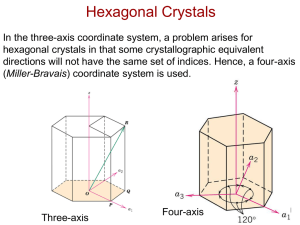
육방 결정의 방향(HCP Crystallographic Directions)
z
방법
a2
-
a3
a1
1. Vector repositioned (if necessary) to pass
through origin.
2. Read off projections in terms of unit
cell dimensions a1, a2, a3, or c
3. Adjust to smallest integer values
4. Enclose in square brackets, no commas
[uvtw]
a
2
Adapted from Fig. 3.8(a),
Callister & Rethwisch 8e.
ex:
½ , ½ , -1, 0
-a3
a2
2
=>
[ 1120 ]
a3
dashed red lines indicate
projections onto a1 and a2 axes
a1
2
a1
Chapter 3 - 27
HCP Crystallographic Directions
• Hexagonal Crystals
– 4개의 축 Miller-Bravais 좌표계에서 방향은 다음과
같은 관계를 갖는다(i.e., u'v'w').
z
[ u 'v 'w ' ] [ uvtw ]
a2
-
a3
a1
1
u = (2 u ' - v ')
3
1
v = (2 v ' - u ')
3
t = - (u +v )
w = w'
Fig. 3.8(a), Callister & Rethwisch 8e.
Chapter 3 - 28
결정면(Crystallographic Planes)
Adapted from Fig. 3.10,
Callister & Rethwisch 8e.
Chapter 3 - 29
결정면(Crystallographic Planes)
• 밀리지수(Miller Indices): 면과 만나는
3좌표축의 역수, 최소의 정수값. 모든 평행한
면은 동일한 밀러지수(reciprocals of the
(three) axial intercepts for a plane, cleared of
fractions & common multiples. All parallel
planes have same Miller indices.)
• Algorithm
1. Read off intercepts of plane with axes in
terms of a, b, c
2. Take reciprocals of intercepts
3. Reduce to smallest integer values
4. Enclose in parentheses, no
commas i.e., (hkl)
Chapter 3 - 30
결정면(Crystallographic Planes)
z
example
1. Intercepts
2. Reciprocals
3.
Reduction
a
1
1/1
1
1
4.
Miller Indices
(110)
example
1. Intercepts
2. Reciprocals
3.
Reduction
a
1/2
1/½
2
2
4.
Miller Indices
(100)
b
1
1/1
1
1
c
1/
0
0
c
y
b
a
x
b
1/
0
0
c
1/
0
0
z
c
y
a
b
x
Chapter 3 - 31
결정면(Crystallographic Planes)
z
example
1. Intercepts
2. Reciprocals
3.
Reduction
4.
Miller Indices
a
1/2
1/½
2
6
b
1
1/1
1
3
(634)
c
c
3/4
1/¾
4/3
4 a
x
y
b
Family of Planes {hkl}
Ex: {100} = (100), (010), (001), (100), (010), (001)
Chapter 3 - 32
결정면[Crystallographic Planes (HCP)]
• In hexagonal unit cells the same idea is used
z
example
1. Intercepts
2. Reciprocals
3.
Reduction
a1
1
1
1
1
a2
1/
0
0
a3
-1
-1
-1
-1
c
1
1
1
1
a2
a3
4.
Miller-Bravais Indices
(1011)
a1
Adapted from Fig. 3.8(b),
Callister & Rethwisch 8e.
Chapter 3 - 33
결정면(Crystallographic Planes)
•
•
결정면의 원자충진
철 포일도 촉매제로 사용이 가능. 노출면의
원자충진이 중요.
a) Fe의 (100)과 (111) 결정 면을 그리시오.
b) 각각의 결정 면에 대한 면밀도를 계산하시오.
Chapter 3 - 34
Virtual Materials Science & Engineering (VMSE)
• VMSE is a tool to visualize materials science topics such as
crystallography and polymer structures in three dimensions
• Available in Student Companion Site at www.wiley.com/college/callister
and in WileyPLUS
Chapter 3 - 35
VMSE: Metallic Crystal Structures &
Crystallography Module
• VMSE allows you to view crystal structures, directions, planes,
etc. and manipulate them in three dimensions
Chapter 3 - 36
Unit Cells for Metals
• VMSE allows you to view the unit cells and manipulate
them in three dimensions
• Below are examples of actual VMSE screen shots
FCC Structure
HCP Structure
Chapter 3 - 37
VMSE: Crystallographic Planes Exercises
Additional practice on indexing crystallographic planes
Chapter 3 - 38
면밀도(Planar Density) of (100) Iron
Solution: At T < 912ºC iron has the BCC structure.
2D repeat unit
(100)
Planar Density =
area
2D repeat unit
1
a2
=
4 3
R
3
Radius of iron R = 0.1241 nm
Adapted from Fig. 3.2(c), Callister & Rethwisch 8e.
atoms
2D repeat unit
a=
1
4 3
R
3
atoms
atoms
19
= 1.2 x 10
2 = 12.1
2
nm
m2
Chapter 3 - 39
Planar Density of (111) Iron
Solution (cont): (111) plane
1 atom in plane/ unit surface cell
2a
atoms in plane
atoms above plane
atoms below plane
h=
3
a
2
2
atoms
2D repeat unit
4 3 16 3 2
2
area = 2 ah = 3 a = 3
R =
R
3
3
1
atoms =
= 7.0
2
Planar Density =
area
2D repeat unit
16 3
3
R
2
nm
0.70 x 1019
atoms
m2
Chapter 3 - 40
VMSE Planar Atomic Arrangements
http://bcs.wiley.com/he-bcs/Books?action=mininav&bcsId=5242&itemId=0470419970&assetId=199053&resourceId=19134
• VMSE allows you to view planar arrangements and rotate
them in 3 dimensions
BCC (110) Plane
Chapter 3 - 41
X-선 회절(X-Ray Diffraction)
• Diffraction gratings must have spacings comparable to
the wavelength of diffracted radiation.
• Can’t resolve spacings
• Spacing is the distance between parallel planes of
atoms.
Chapter 3 - 42
X-Rays to Determine Crystal Structure
• 결정면에 대한 입사 X-선의 회절(Incoming X-rays diffract from crystal planes).
extra
distance
travelled
by wave “2”
q
q
d
Measurement of
critical angle, qc,
allows computation of
planar spacing, d.
reflections must
be in phase for
a detectable signal
Adapted from Fig. 3.20,
Callister & Rethwisch 8e.
spacing
between
planes
X-ray
intensity
(from
detector)
n
d=
2 sin qc
q
qc
Chapter 3 - 43
회절의 조건;
Bragg의 법칙
여기서 면간 거리,
Chapter 3 - 44
Chapter 3 - 45
X-Ray Diffraction Pattern
z
z
Intensity (relative)
c
a
x
z
c
b
y (110)
a
x
c
b
y
a
x (211)
b
(200)
Diffraction angle 2q
Diffraction pattern for polycrystalline -iron (BCC)
Adapted from Fig. 3.22, Callister 8e.
Chapter 3 - 46
y
SUMMARY
• Atoms may assemble into crystalline or
amorphous structures.
• Common metallic crystal structures are FCC, BCC, and
HCP. Coordination number and atomic packing factor
are the same for both FCC and HCP crystal structures.
• We can predict the density of a material, provided we
know the atomic weight, atomic radius, and crystal
geometry (e.g., FCC, BCC, HCP).
• Crystallographic points, directions and planes are
specified in terms of indexing schemes.
Crystallographic directions and planes are related
to atomic linear densities and planar densities.
Chapter 3 - 47
SUMMARY
• Materials can be single crystals or polycrystalline.
Material properties generally vary with single crystal
orientation (i.e., they are anisotropic), but are generally
non-directional (i.e., they are isotropic) in polycrystals
with randomly oriented grains.
• Some materials can have more than one crystal
structure. This is referred to as polymorphism (or
allotropy).
• X-ray diffraction is used for crystal structure and
interplanar spacing determinations.
Chapter 3 - 48
ANNOUNCEMENTS
Reading:
Core Problems:
Self-help Problems:
Chapter 3 - 49