Chapter 3: The Structure of Crystalline Solids
advertisement

Chapter 3: Structures of Metals & Ceramics
ISSUES TO ADDRESS...
• What is the difference in atomic arrangement
between crystalline and noncrystalline solids?
• What features of a metal’s/ceramic’s atomic
structure determine its density?
• How do the crystal structures of ceramic
materials differ from those for metals?
• Under what circumstances does a material
property vary with the measurement direction?
Chapter 3 - 1
Energy and Packing
• Non dense, ________________
Energy
_______________
bond length
typical neighbor
bond energy
• Dense, ___________________
r
Energy
typical neighbor
bond length
r
typical neighbor
bond energy
Dense, _____________________________ to have
_________ energies.
Chapter 3 - 2
Materials and Packing
Crystalline materials...
• atoms pack __________, 3D arrays
• typical of: -________
-many _________
-some _________
crystalline SiO2
Adapted from Fig. 3.41(a),
Callister & Rethwisch 4e.
Noncrystalline materials...
• atoms have no periodic packing
• occurs for: - _________________
- _____________
"Amorphous" = Noncrystalline
Si
Oxygen
noncrystalline SiO2
Adapted from Fig. 3.41(b),
Callister & Rethwisch 4e.
Chapter 3 - 3
Metallic Crystal Structures
• How can we stack metal atoms to minimize
empty space?
2-dimensions
vs.
Now stack these 2-D layers to make 3-D structures
Chapter 3 - 4
Metallic Crystal Structures
• Tend to be densely packed.
• Reasons for dense packing:
- Typically, only one __________is present, so all atomic
________ are the same.
- Metallic bonding is not ________________.
- Nearest neighbor distances tend to be small in
order to __________ bond energy.
- ______________ cloud shields cores from each other
• Metals have the simplest crystal structures.
We will examine three such structures...
Chapter 3 - 5
Simple Cubic Structure (SC)
• Rare due to low packing density (only Po has this structure)
• Close-packed ___________ are cube edges.
• Coordination # = ___
(# nearest neighbors)
Click once on image to start animation
(Courtesy P.M. Anderson)
Chapter 3 - 6
Atomic Packing Factor (APF)
Volume of atoms in unit cell*
APF =
Volume of unit cell
*assume hard spheres
• APF for a simple _______ structure = 0.52
atoms
unit cell
a
R=0.5a
APF =
volume
atom
4
p (0.5a) 3
1
3
a3
close-packed ___________
contains 8 x 1/8 =
1 atom/unit cell
Adapted from Fig. 3.43,
Callister & Rethwisch 4e.
volume
unit cell
Chapter 3 - 7
Body Centered Cubic Structure (BCC)
• Atoms touch each other along cube ____________.
--Note: All atoms are identical; the center atom is shaded
differently only for ease of viewing.
ex: Cr, W, Fe (), Tantalum, ____________
• Coordination # = 8
Click once on image to start animation
(Courtesy P.M. Anderson)
Adapted from Fig. 3.2,
Callister & Rethwisch 4e.
2 atoms/unit cell: 1 center + 8 _____________
Chapter 3 - 8
VMSE Screenshot – BCC Unit Cell
Chapter 3 - 9
Atomic Packing Factor: BCC
• APF for a body-centered _______ structure = 0.68
3a
a
2a
Adapted from
Fig. 3.2(a), Callister &
Rethwisch 4e.
atoms
R
a
4
Close-packed __________:
length = 4R = 3 a
volume
atom
p ( 3a/4) 3
2
unit cell
3
APF =
volume
3
a
unit cell
Chapter 3 - 10
Face Centered Cubic Structure (FCC)
• Atoms touch each other along face ___________.
--Note: All atoms are ____________; the face-centered atoms are shaded
differently only for ease of viewing.
ex: Al, Cu, Au, Pb, Ni, Pt, Ag
• Coordination # = ___
Adapted from Fig. 3.1, Callister & Rethwisch 4e.
Click once on image to start animation
(Courtesy P.M. Anderson)
4 atoms/unit cell: 6 __________ + 8 corners x 1/8
Chapter 3 - 11
Atomic Packing Factor: FCC
• APF for a face-centered cubic structure = 0.74
maximum achievable APF
Close-packed directions:
length = 4R = 2 a
2a
Unit cell contains:
__________________
= __________________
a
Adapted from
Fig. 3.1(a),
Callister &
Rethwisch 4e.
atoms
unit cell
APF =
4
3
p ( 2a/4) 3
volume
atom
volume
unit cell
Chapter 3 - 12
FCC Stacking Sequence
• ABCABC... __________ Sequence
• 2D Projection
B
B
C
A
B
B
B
A sites
C
C
B sites
B
B
C sites
• FCC _____ Cell
A
B
C
Chapter 3 - 13
Hexagonal Close-Packed Structure
(HCP)
• ABAB... Stacking Sequence
• 3D Projection
• 2D Projection
A sites
Top layer
B sites
Middle layer
A sites
Bottom layer
c
a
Adapted from Fig. 3.3(a),
Callister & Rethwisch 4e.
• Coordination # = ___
• APF = _______
• c/a = ________
__ atoms/unit cell
ex: Cd, Mg, Ti, Zn
Chapter 3 - 14
VMSE Screenshot – Stacking Sequence and Unit
Cell for HCP
Chapter 3 - 15
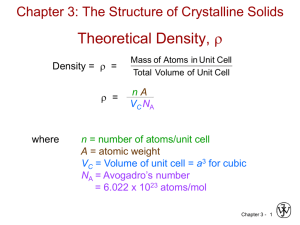
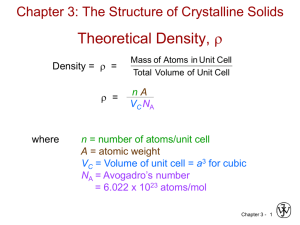
Theoretical Density, r
Density = r =
r =
where
Mass of Atoms in Unit Cell
Total Volume of Unit Cell
nA
VC NA
n = ________________________
A = atomic weight
VC = ________________ = ____________
NA = Avogadro’s number
= 6.022 x 1023 atoms/mol
Chapter 3 - 16
Theoretical Density, r
• Ex: Cr (BCC)
A = 52.00 g/mol
R = ___________
n = 2 atoms/unit cell
Adapted from
Fig. 3.2(a), Callister &
Rethwisch 4e.
atoms
unit cell
r=
volume
unit cell
R
a
52.00
a3 6.022 x 1023
a = 4R/ 3 = 0.2887 nm
g
mol
rtheoretical = 7.18 g/cm3
ractual
atoms
mol
= 7.19 g/cm3
Chapter 3 - 17
Atomic Bonding in Ceramics
• Bonding:
-- ______________________________________.
-- % ionic character __________ with difference in
electronegativity of atoms.
• Degree of ionic character may be large or small:
CaF2: large
SiC: small
Adapted from Fig. 2.7, Callister & Rethwisch 4e. (Fig. 2.7 is adapted from Linus Pauling, The Nature of the
Chemical Bond, 3rd edition, Copyright 1939 and 1940, 3rd edition. Copyright 1960 by
Chapter 3 - 18
Cornell University.)
Ceramic Crystal Structures
Oxide structures
– oxygen anions ________ than metal cations
– close _______ oxygen in a ______ (usually ____)
– cations fit into _______ sites among _______ ions
Chapter 3 - 19
Factors that Determine Crystal Structure
1. Relative sizes of ions – _______________________:
--maximize the # of ___________________________.
-
+
-
-
-
-
_________
2. Maintenance of
Charge Neutrality :
+
-
_______
--_________________
should be zero.
--Reflected in chemical
formula:
CaF 2 :
-
Adapted from Fig. 3.4,
Callister & Rethwisch 4e.
+
-
-
stable
Ca 2+ +
cation
Fanions
F-
A m Xp
m, p values to achieve charge neutrality
Chapter 3 - 20
Coordination # and Ionic Radii
r cation
• __________ # increases with r
anion
To form a ________ structure, how many anions can
surround around a cation?
r cation
r anion
< 0.155
Coord
#
linear
2
0.155 - 0.225
3 ________
0.225 - 0.414
4 tetrahedral
0.414 - 0.732
6 octahedral
0.732 - 1.0
8
Adapted from Table 3.3,
Callister & Rethwisch 4e.
_____
ZnS
(zinc blende)
Adapted from Fig. 3.7,
Callister & Rethwisch 4e.
NaCl
(sodium
chloride)
Adapted from Fig. 3.5,
Callister & Rethwisch 4e.
CsCl
(cesium
chloride)
Adapted from Fig. 3.6,
Callister & Rethwisch 4e.
Chapter 3 - 21
Computation of Minimum Cation-Anion
Radius Ratio
• Determine ________ rcation/ranion for an octahedral site
(C.N. = __)
2ranion + 2rcation = 2a
_________
2ranion + 2rcation = 2 2ranion
ranion + rcation = 2ranion
rcation = ( 2 -1)ranion
rcation
= 2 - 1 = 0.414
ranion
Chapter 3 - 22
Bond Hybridization
Bond Hybridization is possible when there is significant
_________ bonding
– ____________________________
– For example for SiC
•
XSi = 1.8 and XC = 2.5
% ionic character = 100 {1- exp[-0.25(X Si - X C )2 ]} = 11.5%
• ~ 89% ___________ bonding
• Both Si and C prefer sp3 hybridization
• Therefore, for SiC, Si atoms occupy ______________ sites
Chapter 3 - 23
Example Problem: Predicting the Crystal
Structure of FeO
• On the basis of ionic radii, what ________________
would you predict for FeO?
Cation Ionic radius (nm)
Al 3+
0.053
Fe 2+
0.077
Fe 3+
0.069
Ca 2+
0.100
Anion
O2Cl F-
• Answer:
rcation 0.077
=
ranion 0.140
= 0.550
based on this ratio,
-- coord # = ___ because
0.140
0.181
0.133
0.414 < 0.550 < 0.732
-- crystal structure is ____
Data from Table 3.4,
Callister & Rethwisch 4e.
Chapter 3 - 24
Rock Salt Structure
Same concepts can be applied to ____ solids in general.
Example: NaCl (rock salt) structure
rNa = 0.102 nm
rCl = _____ nm
rNa/rCl = ________
cations (Na+) prefer _________ sites
Adapted from Fig. 3.5,
Callister & Rethwisch 4e.
Chapter 3 - 25
MgO and FeO
MgO and FeO also have the NaCl structure
O2-
rO = 0.140 nm
Mg2+
rMg = 0.072 nm
rMg/rO = _______
_________ prefer octahedral sites
Adapted from Fig. 3.5,
Callister & Rethwisch 4e.
So each Mg2+ (or Fe2+) _______ neighbor oxygen atoms
Chapter 3 - 26
AX Crystal Structures
AX–Type Crystal Structures include NaCl, CsCl, and zinc blende
Cesium Chloride structure:
rCs + 0.170
=
= _____
rCl 0.181
Since 0.732 < ____ < 1.0,
_____ sites preferred
Adapted from Fig. 3.6,
Callister & Rethwisch 4e.
So each Cs+ has __ neighbor Cl-
Chapter 3 - 27
AX2 Crystal Structures
Fluorite structure
• Calcium _______ (CaF2)
• Cations in _______ sites
• UO2, ThO2, ZrO2, CeO2
• __________ structure –
positions of cations and
anions reversed
Adapted from Fig. 3.8,
Callister & Rethwisch 4e.
Chapter 3 - 28
ABX3 Crystal Structures
• _________ structure
Ex: complex oxide
_________
Adapted from Fig. 3.9,
Callister & Rethwisch 4e.
Chapter 3 - 29
VMSE Screenshot – Zinc Blende Unit Cell
Chapter 3 - 30
Density Computations for Ceramics
Number of formula units/unit cell
n¢(SAC + SAA )
r=
VC N A
__________ number
Volume of unit cell
SAC = sum of atomic weights of _______________________
SAA= sum of atomic weights of _______________________
Chapter 3 - 31
Densities of Material Classes
In general
rmetals _ rceramics _ rpolymers
30
Why?
20
Metals have...
Ceramics have...
• less _______packing
• often lighter elements
Polymers have...
r (g/cm3 )
• close-packing
10
(metallic bonding)
• often _____atomic masses
• low packing _________
(often _____________)
• lighter elements (C,H,O)
Composites have...
• ______________values
5
4
3
2
1
0.5
0.4
0.3
Metals/
Alloys
Platinum
Gold, W
Tantalum
Silver, Mo
Cu,Ni
Steels
Tin, Zinc
Titanium
Aluminum
Magnesium
Graphite/
Ceramics/
Semicond
Composites/
fibers
Polymers
Based on data in Table B1, Callister
*GFRE, CFRE, & AFRE are Glass,
Carbon, & Aramid Fiber-Reinforced
Epoxy composites (values based on
60% volume fraction of aligned fibers
in an epoxy matrix).
Zirconia
Al oxide
Diamond
Si nitride
Glass -soda
Concrete
Silicon
Graphite
PTFE
Silicone
PVC
PET
PC
HDPE, PS
PP, LDPE
Glass fibers
GFRE*
Carbon fibers
CFRE*
Aramid fibers
AFRE*
Wood
Data from Table B.1, Callister & Rethwisch, 8e.
Chapter 3 - 32
Silicate Ceramics
Most common __________________________
Si4+
O2Adapted from Figs.
3.10-11, Callister &
Rethwisch 4e
crystobalite
• SiO2 (silica) _______________ forms are quartz,
crystobalite, & tridymite
• The strong Si-O bonds lead to a high ____________
temperature (1710ºC) for this material
Chapter 3 - 33
Silicates
Bonding of adjacent SiO44- accomplished by the
sharing of common ___________________
Mg2SiO4
Ca2MgSi2O7
Adapted from Fig.
3.12, Callister &
Rethwisch 4e.
Presence of cations such as Ca2+, Mg2+, & Al3+
1. maintain charge ___________, and
2. ________ bond SiO44- to one another
Chapter 3 - 34
Glass Structure
• Basic Unit:
Glass is
____________(__________)
4Si0 4 tetrahedron
• ____________ is SiO2 to which no
impurities have been added
Si 4+
O2- • Other common _________ contain
impurity ions such as Na+, Ca2+,
Al3+, and B3+
• Quartz is _____________
Na +
SiO2:
4+
Si
O2-
(soda glass)
Adapted from Fig. 3.42,
Callister & Rethwisch 4e.
Chapter 3 - 35
Layered Silicates
• Layered ______ (e.g., clays, mica, talc)
– SiO4 ___________ connected
together to form 2-D plane
• A net negative charge is associated
with each (Si2O5)2- unit
• Negative charge balanced by
_______ plane rich in positively
charged _________
Adapted from Fig.
3.13, Callister &
Rethwisch 4e.
Chapter 3 - 36
Layered Silicates (cont.)
• Kaolinite clay _________ (Si2O5)2- layer with Al2(OH)42+
layer
Adapted from Fig. 3.14,
Callister & Rethwisch 4e.
Note: Adjacent sheets of this type _________ bound to
one another by ________________________.
Chapter 3 - 37
Polymorphic Forms of Carbon
Diamond
– tetrahedral bonding of
carbon
• ______________________
• ______________________
conductivity
– large single crystals –
gem stones
– small ________ – used to
grind/cut other materials
– __________ thin films
• hard surface coatings –
used for cutting tools,
medical devices, etc.
Adapted from Fig. 3.16,
Callister & Rethwisch 4e.
Chapter 3 - 38
Polymorphic Forms of Carbon (cont)
__________
– ______ structure – parallel ___________ arrays of
carbon atoms
Adapted from Fig.
3.17, Callister &
Rethwisch 4e.
– weak van der Waal’s forces between layers
– planes slide easily over one another -- good
lubricant
Chapter 3 - 39
Polymorphic Forms of Carbon (cont)
Fullerenes and Nanotubes
• ___________ – spherical cluster of 60 carbon atoms, C60
– Like a soccer ball
• Carbon __________ – sheet of graphite rolled into a tube
– Ends capped with fullerene _______________
Adapted from Figs.
3.18 & 3.19, Callister
& Rethwisch 4e.
Chapter 3 - 40
Crystals as Building Blocks
• Some engineering applications require ________crystals:
-- diamond single
crystals for abrasives
(Courtesy Martin Deakins,
GE Superabrasives,
Worthington, OH. Used with
permission.)
-- turbine blades
Fig. 9.40(c), Callister &
Rethwisch 4e. (Fig. 9.40(c)
courtesy of Pratt and
Whitney).
• Properties of __________materials
often related to crystal structure.
-- Ex: Quartz fractures more easily
along some crystal planes than
others.
(Courtesy P.M. Anderson)
Chapter 3 - 41
Polycrystals
• Most engineering materials are ____________.
__________
Adapted from Fig. K,
color inset pages of
Callister 5e.
(Fig. K is courtesy of
Paul E. Danielson,
Teledyne Wah Chang
Albany)
1 mm
• Nb-Hf-W plate with an electron beam weld.
• Each _______ is a single crystal.
• If grains are _________ oriented,
Isotropic
overall component properties are not ___________.
• Grain sizes typically range from 1 nm to 2 cm
(i.e., from a few to millions of atomic layers).
Chapter 3 - 42
Single vs Polycrystals
• Single Crystals
E (diagonal) = __________
Data from Table 3.7,
Callister & Rethwisch
4e. (Source of data is
R.W. Hertzberg,
Deformation and
Fracture Mechanics of
Engineering Materials,
3rd ed., John Wiley and
Sons, 1989.)
-Properties vary with
direction: ____________.
-Example: the __________
of elasticity (E) in BCC iron:
• Polycrystals
-Properties may/may not
vary with direction.
-If grains are _________
oriented: __________.
(Epoly iron = 210 GPa)
-If grains are _________,
anisotropic.
E (edge) = 125 GPa
200 mm
Adapted from Fig.
5.19(b), Callister &
Rethwisch 4e.
(Fig. 5.19(b) is courtesy
of L.C. Smith and C.
Brady, the National
Bureau of Standards,
Washington, DC [now
the National Institute of
Standards and
Technology,
Gaithersburg, MD].)
Chapter 3 - 43
Polymorphism
• Two or more distinct _______ structures for the same
material (allotropy/polymorphism)
iron system
titanium
liquid
, -Ti
1538ºC
-Fe
BCC
carbon
1394ºC
__________, graphite
-Fe
FCC
912ºC
BCC
-Fe
Chapter 3 - 44
Crystal Systems
Unit cell: smallest __________________ which
contains the complete ___________ of a
crystal.
7 crystal systems
14 crystal lattices
a, b, and c are the ________ constants
Fig. 3.20, Callister & Rethwisch 4e.
Chapter 3 - 45
Point Coordinates
z
Point coordinates for ________
center are
111
c
a/2, b/2, c/2
y
000
a
x
½½½
b
Point ___________ for unit cell
corner are 111
·
z
2c
·
·
·
b
y
__________: integer multiple of
lattice constants identical
position in another unit cell
b
Chapter 3 - 46
Crystallographic Directions
z
Algorithm
1. Vector ___________ (if necessary) to pass
through origin.
2. Read off ____________ in terms of
unit cell dimensions a, b, and c
y 3. Adjust to smallest ___________ values
4. Enclose in _______ brackets, no commas
x
[uvw]
ex: ___________________________
_______________ where ___________ represents a
negative index
________ of directions <uvw>
Chapter 3 - 47
VMSE Screenshot – [101] Direction
Chapter 3 - 48
Linear Density
• Linear Density of Atoms LD =
Number of atoms
Unit length of direction vector
[110]
ex: linear _________ of Al in [110]
direction
a = 0.405 nm
# atoms
a
Adapted from
Fig. 3.1(a),
Callister &
Rethwisch 4e.
LD =
length
= 3.5 nm-1
2a
Chapter 3 - 49
Drawing HCP Crystallographic Directions (i)
Algorithm (Miller-Bravais coordinates)
1. Remove ____________
2. Divide by largest integer so all values
are ≤ 1
3. Multiply terms by appropriate unit cell
dimension a (for a1, a2, and a3 axes)
or c (for z-axis) to produce
________________
4. Construct _________ by stepping off
these ______________
Adapted from Figure 3.25,
Callister & Rethwisch 4e.
Chapter 3 - 50
Drawing HCP Crystallographic Directions (ii)
• Draw the [1 2 13] ___________in a hexagonal unit cell.
s
a1
a2
a3
z
1. Remove ________ -1
-2
1
3
2
3
1
3
1
Algorithm
Adapted from p. 62,
Callister &
Rethwisch 8e.
2. Divide by 3
[1213]
-
1
3
-
3. ____________
4. Construct Vector
p
r
q
start at point o
proceed –a/3 units along a1 axis to point p
–2a/3 units parallel to a2 axis to point q
a/3 units parallel to a3 axis to point r
c units parallel to z axis to point s
[1213] direction represented by vector from point o to point s
Chapter 3 - 51
Determination of HCP Crystallographic Directions (ii)
Algorithm
1. _________________(if necessary) to pass
through origin.
2. Read off projections in terms of threeaxis (a1, a2, and z) ___________________
a and c
3. Adjust to smallest ________ values
4. Enclose in square brackets, no commas,
for three-axis __________
5. Convert to four-axis Miller-Bravais lattice
coordinates using equations below:
Adapted from p. 74, Callister &
Rethwisch 4e.
u=
1
1
(2u¢ - v ¢) v = (2v ¢ - u¢)
3
3
t = -(u +v)
w = w¢
6. Adjust to smallest integer values and
enclose in brackets [uvtw]
Chapter 3 - 52
Determination of HCP Crystallographic Directions (ii)
Determine indices for green vector
Adapted
from p. 74,
Callister &
Rethwisch
4e.
Example
1. Reposition
2. Projections
3.
Reduction
4.
Brackets
a1
a2
z
not needed
a
a
0c
1
1
0
1
1
0
[110]
5.
6.
Convert to 4-axis parameters
1
1
1
1
u = [(2)(1) - (1)] =
v = [(2)(1) - (1)] =
3
3
3
3
1 1
2
w =0
t = -( + ) = 3 3
3
Reduction & Brackets
1/3, 1/3, -2/3, 0
=>
1, 1, -2, 0
=>
[ 1120 ]
Chapter 3 - 53
Crystallographic Planes
Adapted from Fig. 3.26,
Callister & Rethwisch 4e.
Chapter 3 - 54
Crystallographic Planes
• _____ Indices: Reciprocals of the (three)
axial intercepts for a plane, cleared of
_________ & common multiples. All
________ planes have same Miller indices.
• Algorithm
1. Read off ____________ of plane with axes in
terms of a, b, c
2. Take ____________ of intercepts
3. Reduce to smallest integer values
4. Enclose in parentheses, no
commas i.e., (hkl)
Chapter 3 - 55
Crystallographic Planes
z
example
1. Intercepts
2. Reciprocals
3.
Reduction
a
1
1/1
1
1
4.
Miller Indices
_____
example
1. Intercepts
2. Reciprocals
3.
Reduction
a
1/2
1/½
2
__
4.
Miller Indices
_____
b
1
1/1
1
1
c
__
___
0
__
c
y
b
a
x
b
1/
0
__
c
1/
0
0
z
c
y
a
b
x
Chapter 3 - 56
Crystallographic Planes
z
example
1. Intercepts
2. Reciprocals
3.
Reduction
4.
Miller Indices
a
1/2
1/½
2
6
b
1
1/1
1
3
(634)
c
c
3/4
·
1/¾
4/3
·
4 a
x
·
y
b
Family of Planes {hkl}
Ex: {100} = (100), (010), (001), (100), (010), (001)
Chapter 3 - 57
VMSE Screenshot – Crystallographic Planes
Additional practice on indexing crystallographic planes
Chapter 3 - 58
Crystallographic Planes (HCP)
• In hexagonal unit cells the same idea is used
z
example
1. Intercepts
2. Reciprocals
3.
Reduction
a1
1
1
1
1
a2
1/
0
0
a3
-1
-1
-1
-1
c
1
1
1
1
a2
a3
4.
Miller-Bravais Indices
(1011)
a1
Adapted from Fig. 3.24(b),
Callister & Rethwisch 4e.
Chapter 3 - 59
Crystallographic Planes
•
•
We want to examine the ______ packing of
crystallographic planes
Iron foil can be used as a catalyst. The
atomic packing of the exposed _________
is important.
a) Draw (100) and (111) crystallographic _____ for
Fe.
b) Calculate the planar ________ for each of these
planes.
Chapter 3 - 60
Planar Density of (100) Iron
Solution: At T < 912ºC iron has the ________structure.
2D repeat unit
(100)
Planar Density =
area
2D repeat unit
a2
=
4 3
R
3
__________of iron R = 0.1241 nm
Adapted from Fig. 3.2(c), Callister & Rethwisch 4e.
atoms
2D repeat unit
a=
1
4 3
R
3
atoms
atoms
= ________
2 = ____
2
nm
m2
Chapter 3 - 61
Planar Density of (111) Iron
Solution (cont): (___) plane
______ in plane/ unit surface cell
2a
atoms in plane
atoms above plane
atoms _____ plane
h=
3
a
2
2
atoms
2D repeat unit
4 3 16 3 2
2
area = 2 ah = 3 a = 3
R =
R
3
3
atoms =
= 7.0
2
Planar Density =
area
2D repeat unit
16 3
3
R
2
nm
atoms
__________
m2
Chapter 3 - 62
VMSE Screenshot – Atomic Packing –
(111) Plane for BCC
Chapter 3 - 63
X-Ray Diffraction
• ________ gratings must have spacings comparable to
the wavelength of diffracted ___________.
• Can’t resolve ____________
• Spacing is the distance between __________ planes
of atoms.
Chapter 3 - 64
X-Rays to Determine Crystal Structure
• Incoming X-rays _________ from crystal planes.
extra
distance
travelled
by wave “2”
q
q
d
________________of
critical angle, qc,
allows computation of
planar __________,
d.
reflections must
be in phase for
a detectable signal
Adapted from Fig. 3.38,
Callister & Rethwisch 4e.
spacing
between
_________
X-ray
intensity
(from
detector)
n
d=
2 sin qc
q
qc
Chapter 3 - 65
X-Ray Diffraction Pattern
z
z
Intensity (relative)
c
a
x
z
c
b
y (110)
a
x
c
b
y
a
x (211)
b
(200)
Diffraction angle 2q
Diffraction pattern for polycrystalline -iron (BCC)
Adapted from Fig. 3.40, Callister 4e.
Chapter 3 - 66
y
SUMMARY
• Atoms may assemble into crystalline or amorphous structures.
• Common metallic crystal structures are FCC, BCC, and HCP.
Coordination number and atomic packing factor are the same
for both FCC and HCP crystal structures.
• We can predict the density of a material, provided we know the
atomic weight, atomic radius, and crystal geometry (e.g., FCC,
BCC, HCP).
• Interatomic bonding in ceramics is ionic and/or covalent.
• Ceramic crystal structures are based on:
-- maintaining charge neutrality
-- cation-anion radii ratios.
• Crystallographic points, directions and planes are specified in
terms of indexing schemes. Crystallographic directions and
planes are related to atomic linear densities and planar densities.
Chapter 3 - 67
SUMMARY
• Materials can be single crystals or polycrystalline.
Material properties generally vary with single crystal
orientation (i.e., they are anisotropic), but are generally
non-directional (i.e., they are isotropic) in polycrystals
with randomly oriented grains.
• Some materials can have more than one crystal
structure. This is referred to as polymorphism (or
allotropy).
• X-ray diffraction is used for crystal structure and
interplanar spacing determinations.
Chapter 3 - 68
ANNOUNCEMENTS
Reading:
Core Problems:
Self-help Problems:
Chapter 3 - 69