Hybrid Orbitals & Gas Laws Worksheet Answer Key
advertisement
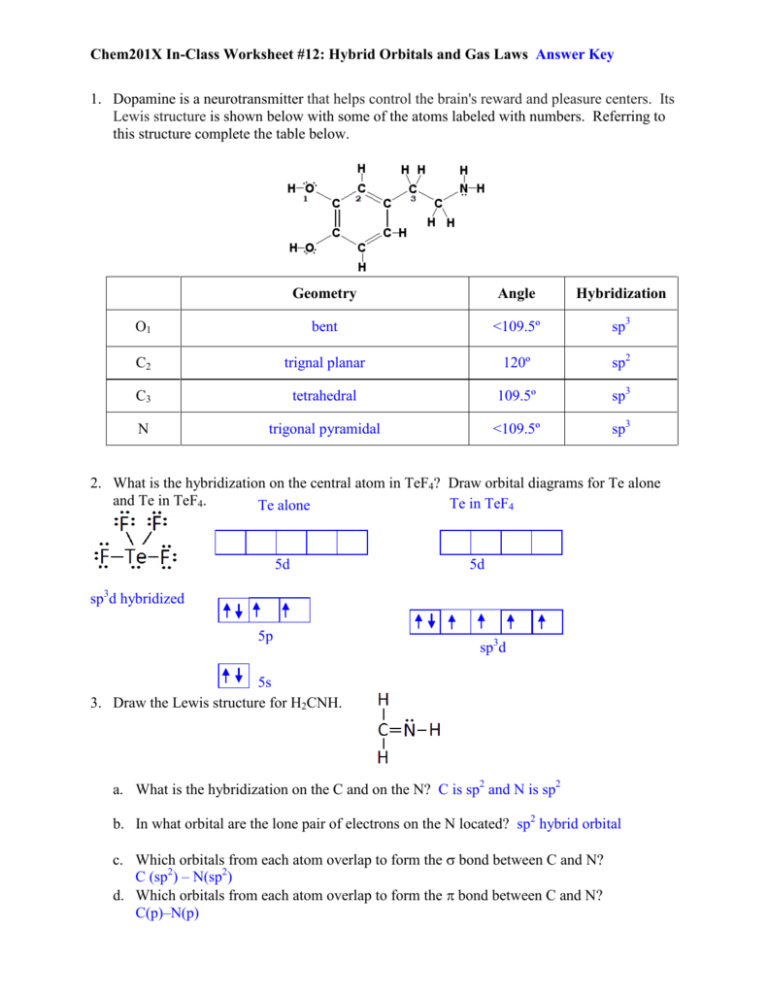
Chem201X In-Class Worksheet #12: Hybrid Orbitals and Gas Laws Answer Key 1. Dopamine is a neurotransmitter that helps control the brain's reward and pleasure centers. Its Lewis structure is shown below with some of the atoms labeled with numbers. Referring to this structure complete the table below. Geometry Angle Hybridization O1 bent <109.5º sp3 C2 trignal planar 120º sp2 C3 tetrahedral 109.5º sp3 N trigonal pyramidal <109.5º sp3 2. What is the hybridization on the central atom in TeF4? Draw orbital diagrams for Te alone and Te in TeF4. Te in TeF4 Te alone 5d 5d sp3d hybridized 5p sp3d 5s 3. Draw the Lewis structure for H2CNH. a. What is the hybridization on the C and on the N? C is sp2 and N is sp2 b. In what orbital are the lone pair of electrons on the N located? sp2 hybrid orbital c. Which orbitals from each atom overlap to form the σ bond between C and N? C (sp2) – N(sp2) d. Which orbitals from each atom overlap to form the π bond between C and N? C(p)–N(p) Chem201X In-Class Worksheet #12: Hybrid Orbitals and Gas Laws Answer Key 4. Aluminum reacts with oxygen gas according to the reaction, 4 Al(s) + 3 O2(g) → 2 Al2O3(s). PV = nRT R = 0.08206 L·atm/mol·K a. How many moles of oxygen are required to react with aluminum to produce 15.2 g of aluminum oxide? (keep all digits of your answer in your calculator for the next part) [0.223617 mol O2] 15.2 g Al2O3 | mol Al2O3 | 3 mol O2 = 0.223617 mol O2 | 101.96 g Al2O3 | 2 mol Al2O3 b. What volume of oxygen gas at 741 mmHg and 19.5 °C will produce 15.2 g of aluminum oxide? [5.51 L] 741 mmHg | 1 atm = 0.975 atm | 760 mmHg PV = nRT 19.5 ºC + 273.15 = 292.65 K V = nRT/V = (0.223617 mol O2)(0.08206 Latm/molK)(292.65 K)/0.975 atm = 5.5078 L = 5.51 L c. If 3.70 g of Al is placed in a 2.15 L container of oxygen at STP (0ºC, 1 atm), assuming a complete reaction, which compound will be the limiting reactant? How many grams of aluminum oxide will be produced? [O2 is LR; 6.52 g Al2O3] 3.70 g Al | mol Al | 2 mol Al2O3 | 101.96 g Al2O3 = 6.99 g Al2O3 | 26.98 g Al | 4 mol Al | 1 mol Al2O3 n = PV/RT = (1 atm)(2.15 L)/[(0.08206 L·atm/mol·K)(273.15 K)] = 0.095919 mol O2 0.095919 mol O2 | 2 mol Al2O3 | 101.96 g Al2O3 | 3 mol O2 | 1 mol Al2O3 = 6.52 g Al2O3 and O2 is LR









