Lab 15: Rate Law of a Reaction using Concentration - Tri
advertisement

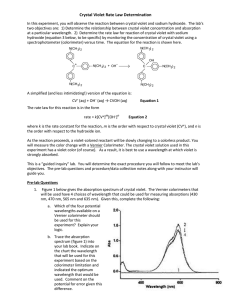
Lab 15: Determining the Rate Law of a Reaction Using Concentration-Time Data -1- Lab 15: Determining the Rate Law of a Reaction using Concentration-Time Data This lab does NOT require a lab report. You will work in groups of 3 or 4 for this lab. I Introduction In this experiment, we will study the kinetics of the decolorization of a dye called crystal violet (a complex molecule often represented simply as CV+). Because of its deep blue color and its affinity for fats, crystal violet is used in biology to stain bacteria and in forensics to stain fingerprints. The structure of CV+, as shown in the net ionic equation below, is relatively complex compared to the compounds we’ve dealt with heretofore. Upon reaction with the hydroxide ion, the CV+ ion is converted to the colorless CVOH compound: + OH- CVOH (Colorless) CV + (Deep blue) Its intense blue color indicates that CV+ should absorb blue’s complementary colors near the opposite end of the spectrum. Indeed, as expected, CV + strongly absorbs wavelengths near 565 nm (verify with the spectrum on page 293 of your textbook that this wavelength is complementary to blue). This strong absorption allows us to easily follow the consumption of CV+ in a reaction (and thus the rate of the reaction) using spectrophotometry (colorimetry). The rate law of the above reaction can be written as - [CV+] t = Rate = k[CV + ] m [OH - ] n where k is the rate constant of the reaction and m and n are the orders of CV+ and OH-, respectively. It is the goal of this lab to calculate k, m, n, and the half-life (t1/2) of this reaction. Lab 15: Determining the Rate Law of a Reaction Using Concentration-Time Data -2- We will perform two trials of the reaction, varying the concentration of hydroxide. Using colorimeters, we will measure the changing concentration of CV+ and then determine the order of reaction, m, with respect to CV+ by plotting the appropriate graphs. We will assume that absorbance is proportional to the concentration of crystal violet (in agreement with Beer’s law). Absorbance will be used in place of concentration in plotting the following three graphs: Absorbance vs. time: A linear plot indicates a zero order reaction (k = – slope). ln Absorbance vs. time: A linear plot indicates a first order reaction (k = – slope). 1/Absorbance vs. time: A linear plot indicates a second order reaction (k = slope). Once m is determined, we will then calculate k, n, and t1/2. In addition, the role of temperature in reaction rates will also be investigated. Different lab groups will use solutions at different temperatures. The rate constants, k, of these different temperatures will be compared to analyze the effect of temperature on reaction rate. Equipment and Reagents LabPro and Colorimeter 50-mL beaker 2 10-mL graduated cylinders Glass stirring rod Water baths at various temperatures 2.50 × 10-5 M crystal violet (CV+) solution 0.10 M NaOH solution 0.050 M NaOH solution ! Warnings! Sodium hydroxide solution is caustic. Avoid spilling it on your skin or clothing. Crystal violet is a biological stain. Avoid spilling it on your skin or clothing. Lab 15: Determining the Rate Law of a Reaction Using Concentration-Time Data 3 Procedure Part 1: Determining m, the order of CV+ Preparing the LabPro and Colorimeter 1. Plug the Colorimeter into Channel 1 of the LabPro and calibrate the colorimeter with a distilled water blank at a wavelength of 565 nm. Empty your blank and dry it with a Q-tip. 2. Set up the data-collection mode. Choose TIME GRAPH mode with a time interval of 4 seconds between data sampling and 45 data samples (points). The total time for data collection will thus be 3 minutes. Return to the main screen. Performing the Reaction 1. Put on your safety goggles: we’re working with strong bases and biological stains. Ugh! 2. Use a 10-mL graduated cylinder to obtain 10.0 mL of 0.10 M NaOH solution from your assigned location (ice bath, hot water bath, or room temperature). Use another 10-mL graduated cylinder to obtain 10.0 mL of 2.5 X 10-5 M crystal violet solution from your assigned location. 3. To initiate the reaction, simultaneously pour the 10-mL portions of crystal violet and sodium hydroxide into a 100-mL beaker and stir the reaction mixture with a stirring rod. Fill your cuvette with the reaction mixture, place it in the colorimeter, and don’t forget to push START! (Speed is not as important in this lab since we’re not using INITIAL rates, but don’t dilly-dally too long.) 4. During the 3-minute data collection, record your observations of the solution in the beaker as it continues to react. 5. After your 3 minutes are up, discard the contents of the beaker and cuvette into the waste beaker at the front of the room. 6. Download your calculator data to my computer, saving it with a descriptive filename to our Common folder. We will analyze and graph the data tomorrow in class using Excel. Part 2: Determining n, the order of OH1. Repeat ALL of the above steps, this time using the 0.050 M NaOH solution. Lab 15: Determining the Rate Law of a Reaction Using Concentration-Time Data 4 Part 3: Analyzing Your Data Determining m, the Order of CV+ 1. Because [OH-] is always much larger (> 5000x) than [CV+], we can consider [OH] to be virtually constant and we can thus rewrite the rate law as a pseudo rate law as follows: [CV+] = Rate = k 1 [CV + ] m t where k1 = k[OH-]n. 2. For Part 1, construct graphs of A(bsorbance) vs. time, ln(A) vs. time, and 1/(A) vs. time to determine m, the order of the reaction with respect to CV +. 3. Add a trendline to your graph, displaying it’s equation and R2 value. From your graph, determine k1. Print your graph. Determining n, the Order of OH1. We can again write a pseudo rate law as follows: - [CV+] t = Rate = k 2 [CV + ] m where k2 = k[OH-]2n. Note that, because [OH-] is different from Part 1, k2 ≠ k1. 2. Now that your know the order of CV+, construct the appropriate graph and determine the rate constant k2, as you did above for k1. 3. Using the values of k1 and k2 and the corresponding OH- concentrations, determine k (including correct units) and n in the following: k1 = k[OH-]1n k2 = k[OH-]2n You have two unknowns and two equations. 4. Using the printed data table, estimate the half-life of the reaction; select two points, one with an absorbance value that is about half of the other absorbance value. The time it takes the absorbance (or concentration) to be halved is known the half-life for the reaction. Then calculate the half-life, t1/2, of this reaction using the equations discussed in class and compare your two values. Lab 15: Determining the Rate Law of a Reaction Using Concentration-Time Data 5 Data and Calculations (to be recorded on your lab Data Sheet) Proce dure and observations (include temperature and LabPro settings) Include printouts of your data tables and graphs from Parts 1 and 2. Include the complete rate law for the decolorization of crystal violet., including orders of reactants and value of k (including units). Include determination of half-life. ? Questions to Answer 1. Identify any sources of error you think you encountered in this lab and explain how they would affect your results (values of k, m, and n). 2. Compare the values of the rate constants, k, for the different temperatures used. In general, as temperature increases, what happens to the rate constant?