KINETICS In this experiment you will find the order of a reaction with
advertisement

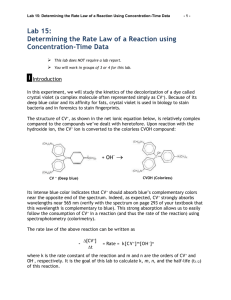
KINETICS In this experiment you will find the order of a reaction with respect to the reactants and calculate the value of the rate constant. The reaction we will be studying is the reaction of a highly colored dye called crystal violet with hydroxide ion. The reaction is shown below: The cationic form of crystal violet (on the reactant side) is purple-violet in color. The neutral form (on the product side) is colorless, as is the hydroxide ion. To simply referring to the two forms, we will use CV+ to represent the colored form and CVOH the colorless form. Part 1: Order of the Reaction With Respect to Crystal Violet In the instantaneous rate method, one experiment is performed and the concentration of one of the chemicals monitored at various times. Plots of [CV+] vs time, ln [CV+] vs time, and 1/[CV+] vs time are used to determined if the reaction is 0, 1st order, or 2nd order.. Since the CV+ is colored and we know the mathematical relationship between [CV+] and Absorbance from the Beer’s Law experiment, we will use a spectrometer to monitor the concentration of the CV+ as the reaction proceeds. The NaOH solution we will use is about 10,000 times more concentrated than the CV+ and will remain essentially constant as the CV+ reacts. This will insure that the rate of the reaction will be dependent only on the remaining concentration of CV+. Ideally, you would do this experiment with the same spectrometer you used in the last experiment. You could then use your Beer’s Law graph to convert the absorbance readings into concentrations so that you can plot the three graphs to determine the order. Since your lab partners have been changed for this experiment it is impractical try to have every new partnership have the spectrometer they used previously. So, to get around this problem we are going to modify the integrated rate law equations. Shown below is a typical Beer’s Law graph from last week’s experiment. The equation of this line is: Considering the error shown for the Y intercept we can say that the value of the Y intercept is effectively equal to 0. So, we can simplify the Beer’s Law line to become: where “m” is the slope of your Beer’s Law graph. Rearranging this equation for [CV] gives: Now lets’s substitute this expression into our three integrated rate law equations. 0 order: If the order is 0, a graph of absorbance vs time would give a straight line with slope equal to -mk. The Y intercept would be the initial absorbance of the solution. 1st order: If the order is 1, a graph of ln(absorbance) vs time will give a straight line with slope equal to -k. The Y intercept would be the natural log of the initial absorbance. 2nd order: If the reaction is 2nd order a graph of 1/abs vs time will give a straight line with slope of k/m and Y intercept that is the reciprocal of the initial absorbance. What these transformations tell us is that when we substitute absorbance for concentration, the same types of graphs we are used to drawing can be used to determine the order of the reaction with respect to crystal violet. Procedure: Warning: In the lab there are bottles of the reactants with different sizes of pipets. Be sure you are using the correct pipet for each trial. Click on the Configure the Spectrometer button in the tool bar (it looks like a graph with a colored spectrum below it). Switch the collection mode to Abs vs time and select a wavelength that is closest to the peak wavelength you observed in your crystal violet spectra from last week. Calibrate the spectrometer with a cuvette of distilled water. Then click on the Data Collection Button (the “clock” to the left of the green button) and change the length of time to 300 seconds and the sampling rate to 10 seconds/sample (not 10 samples/second !!). Click Done to save the setting. Pipet 10.00 mL of crystal violet into a dry, 50 mL beaker. Pipet 10.00 mL of 0.100 M NaOH into a second 50 mL beaker. When you are ready to start the trial pour one of the two solutions into the other AND AT THE SAME TIME start the trial by clicking on the Collect button in the toolbar. Mix the solutions by quickly pouring back and forth between the beakers 5 or 6 times. Quickly fill a dry cuvette with the solution until it is at least 3/4 full. Since the “clock is running” all the time you are doing this try to do these operations as quickly as feasible. Dry off the outside of the cuvette with a tissue and insert it into the spectrometer being careful to orient the cuvette so that the ribbed sides don’t block the light. Leave the cuvette in the spectrometer until the computer stops recording data at 300 seconds. . When the experiment is over, under Experiment select Store Latest Run. This is important to do since it sets up the computer to perform another trial. Discard your solutions down the drain. Part 2: Order of the Reaction With Respect to NaOH Did your remember to “store latest run”? If not and you try to do part 2 the computer will not collect the data until you do, and by then it will be too late since your reaction has already started. In this part of the experiment you will find the order of the reaction with respect to OH-. Since hydroxide ion is colorless, we will need to determine its order in a different way. Pipet 10.00 mL of crystal violet into a dry, 50 mL beaker, and 5.00 mL of 0.100 M NaOH and 5.00 mL of 0.1 M NaCl into a second beaker. In lecture we mentioned that the polarity of the solvent often influences the rate of the chemical reaction and that the polarity of aqueous solutions changes as spectator ions are added. So, to keep the “ionic strength” of the solution constant, the NaCl solution is added to dilute the NaOH instead of just using distilled water. Recalibrate the spectrometer with distilled water. Using another dry cuvette, run a second experiment as you did in Part 1 but using the more dilute NaOH solution. Save your file to your computer. E-mail the Logger Pro file to your partner at this point. Discard your solutions down the drain. Disconnect the spectrometer from your computer. Finding the Order With Respect to Crystal Violet: 1. Calculate the concentrations of NaOH after dilution. 2. If you have a PC, open a second copy of Logger Pro and open your template graph file you prepared during the first lab session that has the three graphs. (If you have a Mac you will probably not be able to open a second Logger Pro so you will need to transfer the data to Excel first and then open up the template graph and copy the data from Excel.) 3. In the template graph file, double click on the Data Set headings and change the name to something that identifies the [NaOH] that was used in the first trial. Change the names in the template file from [A] to Abs, ln[A] to ln(Abs), and 1/[A] to 1/Abs. Double click on the time column and add “seconds” into the units box. 4. The initial points in the graphs contain data that is meaningless since the cuvette was not in the spectrometer yet. Look at data for the trial that had the higher concentration of NaOH. Locate in data the time at which the absorbance readings started to decrease. Copy and paste the times and absorbances after this time into the appropriate places in the table in your template graph file. 5. Linear fit the three graphs in the template file and give them titles. Re-scale as necessary. Enlarge the size of each graph, move the linear fit boxes into blank areas and copy the graphs to the Excel file. The linear graph will allow you to determine the order of the reaction with respect to crystal violet. Finding the Order With Respect to NaOH: 6. In the template graph file, under Data in the toolbar select New Data Set. Change the name of the data set to reflect the [NaOH] in the second trial. Paste the data from your experimental file remembering to start with the point in the data where the absorbance starts to decrease. 7. We want to print the results of both trials on the same set of axes, but just on the graph you have determined to be linear. To add the other set of data, go to Graph Options, Axes Options and select ONE of the variables to be plotted from the second data set. Be sure you are selecting the same variable to be plotted in both data sets. 8. Linear fit the data from the second trial. You should now have two linear graphs on the same set of axes. Add a legend to the graph. 9. Right click on the linear fit boxes and select Linear Fit Options and click on Show Uncertainty. You should now see the expected error in the slope and intercept. 10. Expand the size of the graph and adjust the locations of the various boxes. Change the scaling on both the Y and X axis so that you can see where the two lines cross the Y axis. This is the location where X = 0. You can do this be autoscaling from zero both axes and then “click, hold and drag” the axes as necessary to see the point of interest. If you click on the end of the axis you will drag just that end. Since both trials had the same starting concentration of crystal violet both graphs should intersect the Y axis at the same point. 11. Copy the finished graph to your Excel file. Enter the values of the pseudo rate constants into the table in Excel. Use the uncertainties to determine how many digits you should keep. Finding the Rate Constant: The only things that change the value of the rate constants are T and Ea, neither of which we changed in this experiment. If the two rate constants you just determined are not the same, then they are not the real rate constant. Instead they are “pseudo” rate constants and the order of the reaction with respect to OH- must not be zero In the experiments the [OH-] is at least 1000 times larger than the [CV+] so effectively the [OH-] stays constant during each trial. If [OH-] is effectively constant then the rate law can be written: where kpseudo = k[OH-]y. The “k” we found from the graph is really kpseudo and will be different for each trial if y is not zero since the concentration of hydroxide ion was different in the trials. The values of k (the real rate constant) for the two trials should be the same since we didn’t change the temperature or the activation energy. If we divide the two kpseudo values we get: 12. Use the pseudo rate constants from the linear graphs along with the concentrations of NaOH to calculate the experimental order of the reaction with respect to NaOH. Then round off this experimental value to an integer. 13. To find the real k we will divide kpseudo by [OH-]y. Use the initial concentrations of the hydroxide ion, the integer order with respect to hydroxide, and the pseudo k values to determine the real rate constant. 14. Use the complete rate law along with the balanced equation to decide if the data support the hypothesis that the reaction of crystal violet with NaOH is an elementary step.