Heat Loss Mechanisms - University of Manchester
advertisement
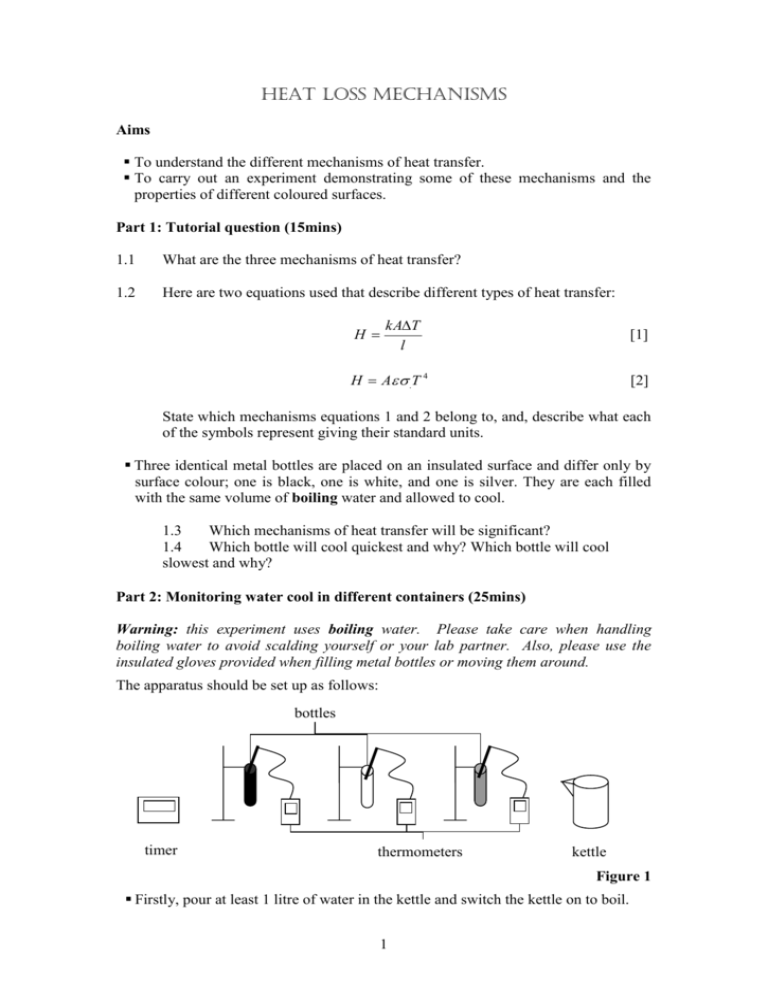
Heat Loss mechanisms Aims To understand the different mechanisms of heat transfer. To carry out an experiment demonstrating some of these mechanisms and the properties of different coloured surfaces. Part 1: Tutorial question (15mins) 1.1 What are the three mechanisms of heat transfer? 1.2 Here are two equations used that describe different types of heat transfer: H kAT l [1] H A .T 4 [2] State which mechanisms equations 1 and 2 belong to, and, describe what each of the symbols represent giving their standard units. Three identical metal bottles are placed on an insulated surface and differ only by surface colour; one is black, one is white, and one is silver. They are each filled with the same volume of boiling water and allowed to cool. 1.3 Which mechanisms of heat transfer will be significant? 1.4 Which bottle will cool quickest and why? Which bottle will cool slowest and why? Part 2: Monitoring water cool in different containers (25mins) Warning: this experiment uses boiling water. Please take care when handling boiling water to avoid scalding yourself or your lab partner. Also, please use the insulated gloves provided when filling metal bottles or moving them around. The apparatus should be set up as follows: bottles timer thermometers kettle Figure 1 Firstly, pour at least 1 litre of water in the kettle and switch the kettle on to boil. 1 Use the plastic measuring jug provided to pour some of the boiling water into each of the three metal bottles to heat them up - funnels are provided to ease the pouring and minimise spillages. Pour all the water from the bottles back into the kettle and switch it on to boil again. You will now fill each bottle with 300ml of boiling water but it is important the starting temperatures of are the same – differing by no more than 2 °C. The following procedure should ensure this for you, if not ask a demonstrator. You should now use the measuring jug and funnel to pour 300ml of boiling water into the silver bottle, then pour 150ml of boiling water into the white bottle, next pour 300ml of water into the black bottle and the final 150ml into the white bottle again. Place a digital thermometer into each of the bottles and seal with caps to reduce heat loss by convection. Start the timer and note the temperatures of the black, white and silver bottles – check that their temperatures are all within 2 °C of each other. Take temperature measurements of each bottle every minute for a total of 20 minutes. These readings should be to the accuracy of the thermometer i.e. one decimal place. Part 2: Analysis (10 mins) 2.1 Plot a graph of the cooling for all three bottles on the one graph. Draw a line (curve) of best fit through each set of points. (Please use different symbols and/or colours to distinguish between the three different bottles). 2.2 Which bottle cooled the slowest? Give an explanation for your answer. Which bottle cooled the fastest? Is this related to the properties of the colour of the bottle or because the starting temperature differed slightly from the rest? 2.3 What is the dominant heat loss process for the bottles? Part 3: (Optional) Take a look at the Crookes Radiometer and observe what happens when light is incident upon one side. 3.1 Can you explain the movement of the “paddles” contained within the vacuum? Hint: One side of the paddle is black and the other is white. Further work The following questions will help in understanding the topic covered by this experimental tutorial. 2 Mastering Physics: Thermal 2: Heat Transfer. There are two questions on “Radiation of Heat” concerned with “Understanding Heat Radiation” and “Heat Radiated by a Person”. Demonstrators' Answers, Hints, Marking Scheme and Equipment List Marking Scheme Section 1.1 1.2 1.3 1.4 2.1 2.2 2.3 Discretionary mark TOTAL Mark 1 1 1 1 2 1 1 2 10 Answers 1.1 Conduction – transfer of heat through a material by transfer of energy from one atom/molecule to its neighbours by a variety of mechanisms (e.g. through lattice vibrations in a solid, movement of conduction electrons in a conductor, exchange of energy via molecular collisions in gases / liquids) Convection – transfer of heat by movement of material, only applies to fluids such as gases and liquids. To model this we need to understand density changes on heating as well as fluid dynamics – not easy to write equations! Radiation – transfer of heat by the emission of electromagnetic radiation – occurs from all objects at all temperatures, but radiated power and wavelength distribution depends on T. 1.2 Conduction [1] H = k A T / l H is heat transferred per second (W) k is the thermal conductivity (W m-1 K-1) T is temperature difference (K) A is the area across which the heat is transferred (m2) l is the length over which the heat is transferred (m) Radiation [2] H = A T4 H is heat transferred per second (W) A is the surface area of the object (m2) is the emissivity (varies from 0 – 1) is Stefan’s constant (W m-2 K-4) 3 T is the absolute temperature in K 1.3 Conduction should be insignificant, due to the low thermal conductivity of air, and the insulated surface. So convection and radiation will play the biggest part. 1.4/1.5 Basically use these two questions to promote a discussion and then prompt students to test their theories by doing the experiment without giving an answer to these latter two questions. 2.1 should see a similar trend to the graph below. 90 88 86 84 82 black 80 silver 78 white 76 74 72 70 0 2 4 6 8 10 2.2 The silver bottle should cool slowest. Silvered surfaces have very low emissivity and so lose relatively little to radiation. 2.3 Accept any reasonable, well explained answer. The black bottle should cool fastest. Black has the highest emissivity, e, of ~1 and so loses the most to radiation. However, emissivities of different paints and coatings, whilst varying widely at optical wavelengths are mostly similar at infrared wavelengths. The peak of the blackbody spectrum at 90 °C = 363 K is at a wavelength of about 8 microns, much longer than optical wavelengths of about 0.3-0.7 microns. For 8 microns wavelength, we would expect emissivity values in the range of about 0.8-0.9 for common anodized aluminium coatings, along with many other paints. Plain aluminium at this wavelength has an emissivity of about 0.03. So a clear difference between aluminium and the paints should be seen, however detecting differences between the black and white coatings will be difficult if at all possible. 4 2.4 The dominant heat loss process for the bottles is convection – the differences in radiative losses due to differences in emissivity were small relative to the overall cooling rate, the overall cooling rate must therefore have been dominated by the other main process – convection. 3. Crooke’s Radiometer. Not as straightforward as you might first think – note it rotates the wrong way for it being radiation pressure ! The following is from Wikipedia. Over the years, there have been many attempts to explain how a Crookes radiometer works: 1. Crookes incorrectly suggested that the force was due to the pressure of light. This theory was originally supported by James Clerk Maxwell who had predicted this force. This explanation is still often seen in leaflets packaged with the device. The first experiment to disprove this theory was done by Arthur Schuster in 1876, who observed that there was a force on the glass bulb of the Crookes radiometer that was in the opposite direction to the rotation of the vanes. This showed that the force turning the vanes was generated inside the radiometer. If light pressure was the cause of the rotation, then the better the vacuum in the bulb, the less air resistance to movement, and the faster the vanes should spin. In 1901, with a better vacuum pump, Pyotr Lebedev showed that in fact, the radiometer only works when there is low pressure gas in the bulb, and the vanes stay motionless in a hard vacuum. Finally, if light pressure were the motive force, the radiometer would spin in the opposite direction as the photons on the shiny side being reflected would deposit more momentum than on the black side where the photons are absorbed. The actual pressure exerted by light is far too small to move these vanes but can be measured with devices such as the Nichols radiometer. 2. Another incorrect theory was that the heat on the dark side was causing the material to outgas, which pushed the radiometer around. This was effectively disproved by both Schuster's and Lebedev's experiments. 3. A partial explanation is that gas molecules hitting the warmer side of the vane will pick up some of the heat i.e. will bounce off the vane with increased speed. Giving the molecule this extra boost effectively means that a minute pressure is exerted on the vane. The imbalance of this effect between the warmer black side and the cooler silver side means the net pressure on the vane is equivalent to a push on the black side, and as a result the vanes spin round with the black side trailing. The problem with this idea is that the faster moving molecules produce more force, they also do a better job of stopping other molecules from reaching the vane, so the force on the vane should be exactly the same — the greater temperature causes a decrease in local density which results in the same force on both sides. Years after this explanation was dismissed, Albert Einstein showed that the two pressures do not cancel out exactly at the edges of the vanes because of the temperature difference there. The force predicted by Einstein would be enough to move the vanes, but not fast enough. 5 4. The final piece of the puzzle, thermal transpiration, was theorized by Osborne Reynolds, but first published by James Clerk Maxwell in the last paper before his death in 1879. Reynolds found that if a porous plate is kept hotter on one side than the other, the interactions between gas molecules and the plates are such that gas will flow through from the cooler to the hotter side. The vanes of a typical Crookes radiometer are not porous, but the space past their edges behave like the pores in Reynolds's plate. On average, the gas molecules move from the cold side toward the hot side whenever the pressure ratio is less than the square root of the (absolute) temperature ratio. The pressure difference causes the vane to move cold (white) side forward. Both Einstein's and Reynolds's forces appear to cause a Crookes radiometer to rotate, although it still isn't clear which one is stronger. 6 Equipment List: 3 x bottles (black, white, silver) 3 x thermometers Kettle Timer Foil Bottle rack 3 x different coloured pencils 7









