Theme : PLANT WATER REGIME
advertisement
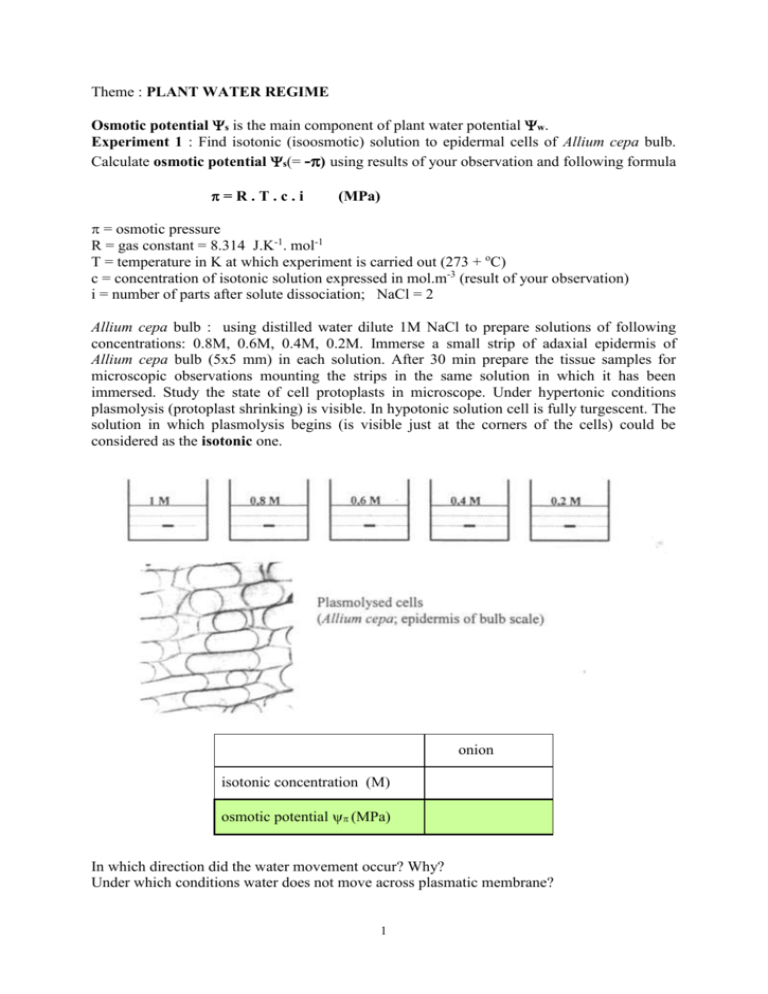
Theme : PLANT WATER REGIME Osmotic potential s is the main component of plant water potential w. Experiment 1 : Find isotonic (isoosmotic) solution to epidermal cells of Allium cepa bulb. Calculate osmotic potential s(= -) using results of your observation and following formula =R.T.c.i (MPa) = osmotic pressure R = gas constant = 8.314 J.K-1. mol-1 T = temperature in K at which experiment is carried out (273 + oC) c = concentration of isotonic solution expressed in mol.m-3 (result of your observation) i = number of parts after solute dissociation; NaCl = 2 Allium cepa bulb : using distilled water dilute 1M NaCl to prepare solutions of following concentrations: 0.8M, 0.6M, 0.4M, 0.2M. Immerse a small strip of adaxial epidermis of Allium cepa bulb (5x5 mm) in each solution. After 30 min prepare the tissue samples for microscopic observations mounting the strips in the same solution in which it has been immersed. Study the state of cell protoplasts in microscope. Under hypertonic conditions plasmolysis (protoplast shrinking) is visible. In hypotonic solution cell is fully turgescent. The solution in which plasmolysis begins (is visible just at the corners of the cells) could be considered as the isotonic one. onion isotonic concentration (M) osmotic potential (MPa) In which direction did the water movement occur? Why? Under which conditions water does not move across plasmatic membrane? 1 Experiment 2 : Experiment 2a: Simulate water transport in vascular tissues of sunflower using colour inks, follow time course and localisation of water (ink) transport. Place 2 test tubes in the rack so that the necks will be in contact. Put in each test tube ink of different colour. Cut the plant with blade 5 cm above the substrate and transfer the cutting area as fast as possible into a vessel filled with water. Further manipulate with the plant material under water. Carry out 5 cm long longitudinal section in the middle of the stem. Each half of the stem place into one test tube and attach test tubes together. Put the plant under direct light (table lamp) and follow transport of the ink along the stem. Scheme of experiment Experiment 2b: Detect lignin in vascular tissues on transversal stem and petiole sections of sunflower. Prepare several thin transversal sections of another plant (node and internode stem and petiole sections) and put them into a drop of HCl-floroglucinol. After 5 min carry the sections into a drop of acid glycerol on a slide, cover with cover-glass and observe under microscope. Draw a schematic picture and explain where lignin was detected and why. Similarly prepare transversal sections of plants used in Experiment 2a and evaluate under microscope, do not use the dye. In which direction did the water movement occur? Why? What could happen if the longitudinal stem sections have not been carried out under water? Did the colour inks mix together in the plant tissues? Experiment 3: Follow transpiration flow and root pressure of sunflower plants. Cut the plant with blade 5 cm above the substrate and transfer the cutting area as fast as possible into a vessel filled with water. The rest of the plant will be used for measurement of root pressure (see below). Bind the rubber tube to the capillary, fill them with water and put the plant into the rubber tube under water so that the system is all the time fully irrigated. Make sure that all parts of the system are tightly in contact and attach to the stand according to the picture. 2 Scheme of the experiment Put the plant under direct light (table lamp) and mark the starting level in the capillary. Every 10 min mark the level of water column. Measure the height of water column for each time and prepare a graph of water decrease (ml) depending on time. Calculate transpiration rate in ml/min. On the stump of the plant put a rubber tube mounted to a capillary and attach to the stand according to the picture. Mark the starting level in the capillary. Every 10-15 min mark the level of water column. Measure the height of water column for each time and calculate root pressure in ml/min. Scheme of the experiment 3 Calculate transpiration rate and try to describe the relationship between this parameter and environmental conditions. Explain this relationship. Calculate root pressure and explain its dependence on osmotic status of cultivation conditions. 4











