MOLE
advertisement
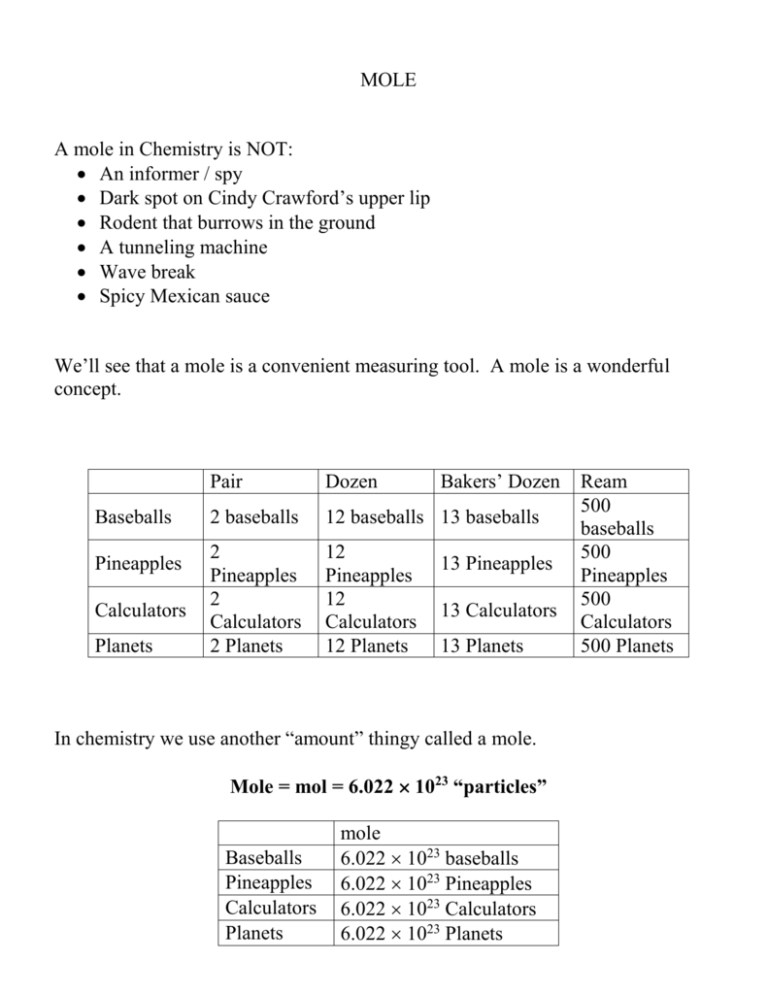
MOLE A mole in Chemistry is NOT: An informer / spy Dark spot on Cindy Crawford’s upper lip Rodent that burrows in the ground A tunneling machine Wave break Spicy Mexican sauce We’ll see that a mole is a convenient measuring tool. A mole is a wonderful concept. Baseballs Pineapples Calculators Planets Bakers’ Dozen Pair Dozen 2 baseballs 12 baseballs 13 baseballs 2 Pineapples 2 Calculators 2 Planets 12 Pineapples 12 Calculators 12 Planets 13 Pineapples 13 Calculators 13 Planets In chemistry we use another “amount” thingy called a mole. Mole = mol = 6.022 1023 “particles” Baseballs Pineapples Calculators Planets mole 6.022 1023 baseballs 6.022 1023 Pineapples 6.022 1023 Calculators 6.022 1023 Planets Ream 500 baseballs 500 Pineapples 500 Calculators 500 Planets Just as 1 doz eggs = 12 eggs We can also say 1 mol of eggs = 6.022 1023 eggs A mole is a “Number” 1 mol of H = 6.022 1023 atoms of H 1 mol of O = 6.022 1023 atoms of O 1 mol of Al = 6.022 1023 atoms of Al 1 mol of Cr = 6.022 1023 atoms of Cr We use moles in chemistry as a way to COUNT what we have. However, In chemistry, the mol is more then just a number. The mole concept was developed alongside atomic mass: 1 mol amu = 1.00 g 1amu 1 mass 12 C 1.661 10 24 g 12 Why use 1/12 mass of a 12C and call it an “amu”----12C makes a nice standard---- 1 amu is equal to the average mass of a proton and a neutron (electrons are relatively mass-less compared to the proton or neutron so we ignore the mass of an electron)…so if you add 12 amu’s you get the mass of a carbon (which is equal to 6 protons and 6 neutrons…that is where the 12 amu’s comes from…6 protons plus 6 neutrons)….16 amu’s you get the mass of an oxygen atom (8 protons plus 8 neutrons is 16)….52 amu’s gives you the mass of a chromium atom (26 protons plus 26 neutrons equals 52). The entire periodic table is based on it’s relative mass as compared to 12C. 1 mol amu = 1.00 g mole = a big number AND mass Proof: 1 mol = 6.022 1023 amu = 1.661 10-24 g 1 mol amu = (6.022 1023) (1.661 10-24g) = 1.00 g Therefore, a mole of an element = elements amu expressed in g------More Simply: 1 mol of H = 1 amu = 1 g H 1 mol of O = 16 amu = 16 g O 1 mol of Al = 27 amu = 27 g Al 1 mol of Cr = 52 amu = 52 g Cr “Key-Point”-----mol in chemistry is a function of numbers to mass….Beauty of a mol is…..by weighing something you know how many atoms you have. This is the only way chemists have to relate numbers (number of atoms of one compound) to mass (how much of the other atoms do I need in the reaction) in ALL CHEMICAL EQUATIONS. All the information we need is conveniently laid out on the Periodic Table. Question what weighs more a mole of H or a mole of Cr? Which has more atoms? MOLECULAR WEIGHT & FORMULA WEIGHT Note: that Molecular Weight and Molecular Mass mean the same thing (as you may remember we said that mass weight…that weight had gravity associated with it…see Chapter 1 subheading Matter). In fact Formula Weight and Formula Mass mean the same thing. Chemist have never changed these terms to reflect what they should mean. I use mw = molecular weight. A mole of Fe = Fe 55.85 amu = Fe 55.85 g AMU -VS- MW amu is replaced with mw to indicate we are speaking in terms of molecules. AMU refers to elements and MW refers to molecules or collection of atoms (i.e. collection of AMU’s). 55.85 g Fe 1moleFe 55.85amuFe g 55.85 55.85amu mol FeSO4 is a molecule containing iron. A molecule is recorded as MW, hence 1moleFeSO4 151.90 g FeSO4 151.90mw FeSO4







