What are Polycyclic Aromatic Hydrocarbons
advertisement
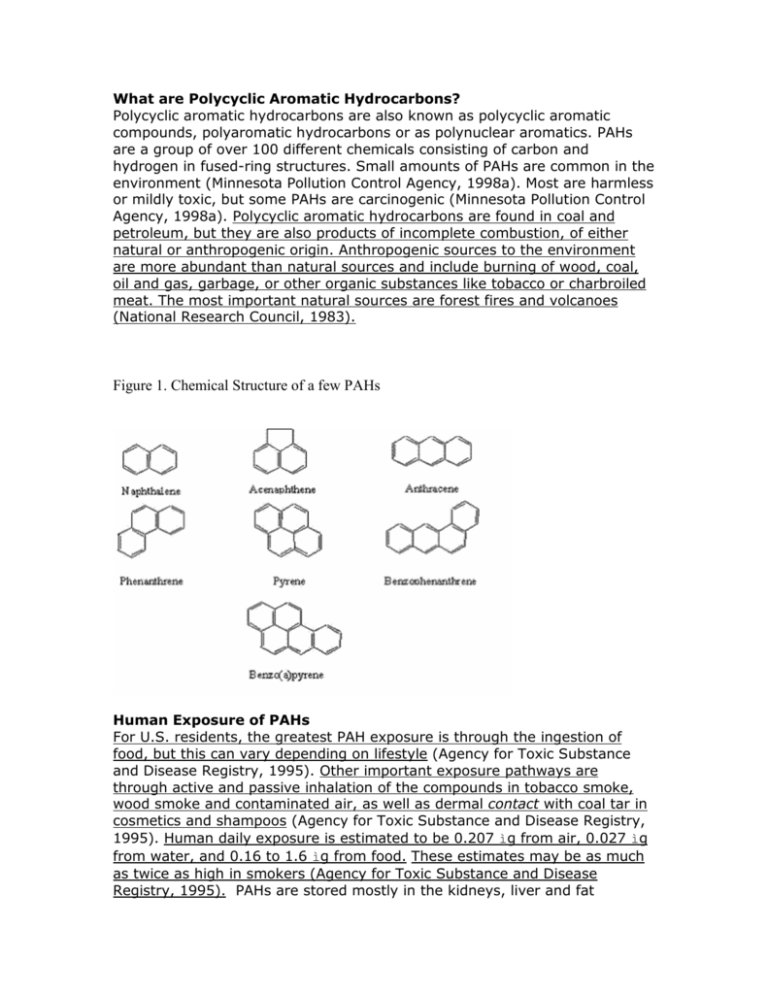
What are Polycyclic Aromatic Hydrocarbons? Polycyclic aromatic hydrocarbons are also known as polycyclic aromatic compounds, polyaromatic hydrocarbons or as polynuclear aromatics. PAHs are a group of over 100 different chemicals consisting of carbon and hydrogen in fused-ring structures. Small amounts of PAHs are common in the environment (Minnesota Pollution Control Agency, 1998a). Most are harmless or mildly toxic, but some PAHs are carcinogenic (Minnesota Pollution Control Agency, 1998a). Polycyclic aromatic hydrocarbons are found in coal and petroleum, but they are also products of incomplete combustion, of either natural or anthropogenic origin. Anthropogenic sources to the environment are more abundant than natural sources and include burning of wood, coal, oil and gas, garbage, or other organic substances like tobacco or charbroiled meat. The most important natural sources are forest fires and volcanoes (National Research Council, 1983). Figure 1. Chemical Structure of a few PAHs Human Exposure of PAHs For U.S. residents, the greatest PAH exposure is through the ingestion of food, but this can vary depending on lifestyle (Agency for Toxic Substance and Disease Registry, 1995). Other important exposure pathways are through active and passive inhalation of the compounds in tobacco smoke, wood smoke and contaminated air, as well as dermal contact with coal tar in cosmetics and shampoos (Agency for Toxic Substance and Disease Registry, 1995). Human daily exposure is estimated to be 0.207 ìg from air, 0.027 ìg from water, and 0.16 to 1.6 ìg from food. These estimates may be as much as twice as high in smokers (Agency for Toxic Substance and Disease Registry, 1995). PAHs are stored mostly in the kidneys, liver and fat (Agency for Toxic Substance and Disease Registry, 1995). In long-term PAH exposure scenarios, cancer, cataracts, kidney and liver damage, and jaundice may develop. However, in humans most PAHs are released in feces and urine within a few days (Agency for Toxic Substance and Disease Registry, 1995). The U.S. Food and Drug Administration states that over-the-counter products with coal tar concentrations between 0.5% and 5% are safe. The FDA maintains that there is no evidence that coal tar products cause cancer (National Psoriasis Foundation, 2005). The Environment and PAHs In the environment PAHs can volatilize, photolyze, oxidize, biodegrade, bind to suspended particles, or accumulate in aquatic organisms (Agency for Toxic Substance and Disease Registry, 1995). The bulk of PAHs in the environment is tied to organic matter in soil. The Air and PAHs Most PAHs, whether the result of natural or anthropogenic processes, are released into the air. Residential burning of wood is the largest anthropogenic source, however on a local scale other emission sources can be dominant (Peters et al., 1981; Ramdahl et al., 1982). Urban air contains as much as 5 times higher PAH concentrations (0.15-19.3 ng/m3) than rural air (0.02-1.2 ng/m3) (Agency for Toxic Substance and Disease Registry, 1995). Winter concentrations are 5-10 times higher than summer concentrations due to the difference in temperature, sunlight radiation, and an increase in heating emissions (Agency for Toxic Substance and Disease Registry, 1995). An estimated 10,000 to 30,000 tons (8,900- 26,800 metric tons) of PAHs are emitted annually in the U.S. (Baek et al., 1991; EPA, 1998). The Water and PAHs The most important source of PAHs in surface water is from the deposition of airborne PAHs (Jensen, 1984). Other sources include municipal waste water discharge (Barrick, 1982), urban storm water runoff (MacKenzie and Hunter, 1979), runoff from coal storage areas (Stahl et al., 1984; Wachter and Blackwood, 1979), effluents from wood treatment plants and other industries (DeLeon et al., 1986; Snider and Manning, 1979; United States Department of Agriculture, 1978), oil spills, and petroleum processing (Giger and Blumer, 1974). In a localized environment any of the above sources can be dominant. Background levels of PAHs in drinking water range from 4 to 24 ng/L (Sorrel et al., 1980). The Soil and PAHs Soil, like water, receives most PAHs from atmospheric deposition after local and long-range transport (Agency for Toxic Substance and Disease Registry, 1995). Other sources include sludge disposal from public sewage treatment plants, automotive exhaust, tire and asphalt wear, irrigation with coke oven effluent, leachate from bituminous coal storage sites, and use of compostbased fertilizers (Perwak et al., 1982; Santodonato, 1981; Stahl et al., 1984; White and Lee, 1980).











