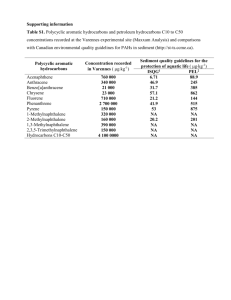
Multi-pathway assessment of human health risk posed by polycyclic aromatic hydrocarbons
advertisement

Environ Geochem Health (2015) 37:587–601 DOI 10.1007/s10653-014-9675-7 ORIGINAL PAPER Multi-pathway assessment of human health risk posed by polycyclic aromatic hydrocarbons Changsheng Qu • Bing Li • Haisuo Wu Shui Wang • John P. Giesy • Received: 8 November 2014 / Accepted: 30 December 2014 / Published online: 9 January 2015 Ó Springer Science+Business Media Dordrecht 2015 Abstract To assess aggregate exposure to polycyclic aromatic hydrocarbons (PAHs) via several environmental media and pathways, a probabilistic framework for multi-pathway health risk assessment that integrates PAHs potency equivalence factors, risk estimation modeling, and Monte Carlo simulation was applied to a case study in Nanjing, which is an important industrial city in China. Incremental lifetime risk of additional cancers posed by exposure to 16 USEPA priority PAHs in air, water, soil, and fish was assessed. Risks to three age groups, infants, children, and adults, through various exposure pathways, including oral ingestion, dermal absorption, and inhalation, were estimated. Results of the analysis of risk indicated that B[a]P, B[b]F, and BA were the predominant PAHs pollutants in Nanjing. Risk of additional cancer for local adults was on average 2.62 9 10-5. The risks were primarily due to ingestion of fish and inhalation, which contributed 99 % of the total risks. By contrast, risk to infants was essentially negligible. Results of a sensitivity analysis indicated that the input variables of concentration of PAHs in fish (Cf), the body weight (BW), and the ingestion rate of fish (IRf) were the major influences on estimates of risks. C. Qu (&) B. Li H. Wu S. Wang Key Laboratory of Environmental Engineering, Jiangsu Academy of Environmental Science, Nanjing 210036, China e-mail: 031202026@163.com J. P. Giesy Department of Zoology, Center for Integrative Toxicology, Michigan State University, East Lansing, MI, USA J. P. Giesy State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing 210023, China J. P. Giesy Department of Veterinary Biomedical Sciences, Toxicology Centre, University of Saskatchewan, Saskatoon, SK, Canada Keywords PAHs Environmental exposure Multipathway Monte Carlo simulation Asia Cancer Introduction Polycyclic aromatic hydrocarbons (PAHs) are a group of organic compounds containing two or more fused J. P. Giesy Department of Biology and Chemistry and State Key Laboratory in Marine Pollution, City University of Hong Kong, Kowloon, Hong Kong, SAR, China J. P. Giesy School of Biological Sciences, University of Hong Kong, Hong Kong, SAR, China 123 588 aromatic rings. They can enter into and spread through the environment via various routes, including domestic and industrial wastewater discharges, oil spills, tire wear debris, asphalt particles, atmospheric transport, dispersion and deposition of industrial stack emission, and vehicle exhaust (Binet et al. 2002; Srogi 2007; Feng et al. 2009). Because of their potential to bioaccumulate, persistence, and carcinogenic and mutagenic potencies, PAHs have been of scientific interest for many years (Orecchio and Papuzza 2009). Hundreds of these compounds exist in the environment, 16 of which with greater toxicities have been selected by the US Environmental Protection Agency (USEPA) as priority pollutants to be controlled and have, therefore, been routinely analyzed (Sun et al. 1998) (Table 1). In China, since the 1970s, rapid development of industry, agriculture, and municipalities has resulted in greater loads of PAHs being distributed in the atmosphere, water, and soil (Xu et al. 2006; Shi et al. 2011; Zhang et al. 2014). In the past few decades, previous studies have focused on the occurrence, sources, and spatial distribution of PAHs (Feng et al. 2009; Wang et al. 2011a, b; Cao et al. 2010; Lin et al. 2012; Zhang et al. 2011). It was found that Chinese PAH emissions contributed over 20 % to the total global PAH emissions (Zhang and Tao 2009). As a result, PAH emissions in excess of 116,000 tons/year have resulted in the contamination of various environmental media in China (Zhang et al. 2007). For instance, on the whole, ranking concentration levels of PAHs in rivers of the world, PAHs level in water-dissolved phase in Chinese rivers was a little higher than that in other countries (Feng et al. 2009). These researches currently serve many compliance monitoring needs. However, PAHs can pose risk to the public through inhalation of dust and from soil and water by direct ingestion and dermal contact. Because of the rapid increase in automobile and industrial production, the general Chinese population has more opportunities to be exposed to PAHs from multiple sources and routes than do people in most other places in the world (Ji et al. 2010). It was reported by 1.6 % of the lung cancer morbidity in China was due to inhalation exposure to ambient air PAHs (Zhang et al. 2009). Therefore, to protect public health, besides environmental monitoring studies, potential exposures to these PAHs and their associated health risks deserve more attention. Assessment of risks to human health, formalized in 1983 by the US National Research Council, is a process 123 Environ Geochem Health (2015) 37:587–601 for estimating potential adverse health effects to humans from exposure to chemicals in environmental media (NRC 1983a). Several studies have been conducted to evaluate risks posed by exposure to PAHs through different environmental media or exposure pathways. According to the risk assessment results, the occupational risk to traffic policemen in Tianjin fell within the range from 10-6 to 10-3 through inhalation (Hu et al. 2007), the cancer risk levels via dermal contact and ingestion of soil ranged from 10-7 to 10-6 in Guangzhou (Wang et al. 2011a, b), and the average cancer risk caused by dietary exposure fell within the range from 10-6 to 10-5 in Taiyuan (Xia et al. 2010). It can be found that oral ingestion and dermal contact of soil and food, as well as inhalation, may all pose potential risk to the public. However, most of previous reports focused on exposure to PAHs through certain environmental media or exposure pathways. Studies concerning multi-pathway assessment of human health risk are quite limited. A media- or pathway-specific assessment approach might not guarantee public safety, for the public expose to PAHs through various environmental media or exposure pathways. It is difficult to derive the overall health risk and identify the key exposure route without multi-pathway risk analysis. Therefore, it is vital to assess aggregate exposure to PAHs via multiple media and pathways (Qu et al. 2012a; Marin et al. 2003). In this study, an integrated, multi-pathway assessment to estimate the lifetime risk of additional cancers due to exposure to PAHs through multiple pathways, including oral ingestion, dermal contact, and inhalation, was conducted in Nanjing. As an extension of previous efforts, the published literature on concentrations of the 16 EPA priority PAHs in air, water, soil, and food in Nanjing was reviewed. Next, a multi-pathway assessment of risks of additional cancers was conducted to fill the gap between routine environmental monitoring data and the decision making support for protecting populations from exposure to PAHs. Detailed sensitivity and uncertainty analyses were conducted to identify the critical input variables that require further study. Materials and methods Study area Nanjing, a typical megacity in eastern China (31° and 32°N, 118° and 119°E) with more than 8.1 million 2.1–6.7 1.8–11 0.5–2.0 1.0–9.4 Benzo[a]pyrene (B[a]P) Indeno[1,2,3-c,d]pyrene (InP) Dibenz[a,h]anthracene (DBA) Benzo[g,h,i]perylene (BghiP) ND not detected 14–43 ND Benzo[b]fluoranthene (BbF) Benzo[k]fluoranthene (BkF) 3.3–8.9 Chrysene (Chr) 5.7 ± 2.4 1.5–9.1 ND–13 0.3 ± 0.7 2.1 ± 2.6 ND–1.9 0.8–7.9 Anthracene (Ant) Fluoranthene (Flt) Pyrene (Pyr) ND 0.3 ± 0.7 ND ND–1.8 Fluorene (Flu) Phenanthrene (Phe) Benz[a]anthracene (BA) ND ND Acenaphthene (Ace) 4.0 ± 2.7 0.9 ± 0.5 4.4 ± 1.6 5.5 ± 3.1 ND 29 ± 11 6.7 ± 1.9 4.0 ± 4.2 ND ND ND ND ND ND ND ND ND ND 0.06–2.11 0.05–0.73 0.06–2.61 ND–1.33 ND–0.58 ND–0.99 ND ND ND ND Range Mean Range Naphthalene (Nap) Water (ng/L) Air (ng/m3) Acenaphthylene (Ac) Compounds Table 1 Concentrations of PAHs in air, water, and soil in Nanjing ND ND ND ND ND ND ND ND ND ND 0.19 ± 0.88 0.13 ± 0.28 0.22 ± 1.08 0.44 ± 0.53 0.19 ± 0.23 0.28 ± 0.41 Mean ND–11.67 1.32–8.89 0.70–5.26 ND–20.08 0.79–5.87 1.57–51.67 0.67–47.58 0.92–7.64 0.90–10.77 3.56–78.60 1.03–7.07 2.61–54.46 ND–22.23 ND ND ND–89.90 Range Soil (ng/g) 3.43 ± 3.17 3.34 ± 2.50 2.00 ± 1.45 3.33 ± 5.68 1.90 ± 1.50 19.85 ± 14.60 13.00 ± 12.02 3.72 ± 3.46 2.61 ± 2.80 24.55 ± 21.96 2.70 ± 2.14 24.08 ± 17.06 8.68 ± 7.34 ND ND 32.92 ± 27.46 Mean 0.1–15.6 0.1–1.5 0.3–17.4 0.1–9.3 0.1–9.1 0.2–13.5 4.4–132 1.2–55.6 22.4–267 43.4–509 9.5–611 161–794 28–320 5.2–207 3.5–30.7 – Range Fish (ng/g) 0.87 ± 3.01 0.17 ± 0.30 1.04 ± 3.36 0.57 ± 1.81 1.18 ± 2.00 2.00 ± 3.02 26.50 ± 26.74 4.70 ± 10.73 101.46 ± 68.02 204.97 ± 122.63 39.84 ± 116.71 422.67 ± 179.77 92.40 ± 71.45 19.60 ± 40.77 9.67 ± 6.06 – Mean Environ Geochem Health (2015) 37:587–601 589 123 590 Environ Geochem Health (2015) 37:587–601 Fig. 1 Location of the study area residents, is the capital of Jiangsu Province and located in the lower Yangtze River drainage basin (Fig. 1). As one of the most rapidly developing megalopolises of China, Nanjing has experienced accelerated industrialization and urbanization in recent years. At the same time, PAHs are being emitted to the environment, where they might pose a threat to the public. Because contamination by the PAHs is an increasing environmental concern, the comprehensive study of human exposure to the PAHs pollutants in Nanjing deserves much more attention. performance liquid chromatography (HPLC) (Yin et al. 2008). Little information on concentrations of PAHs in food of Nanjing was available. Since fish protein features prominently in the diet of people in Nanjing, the concentration data of PAHs in fish were used to assess this risk posed by ingestion of food. A total of 193 samples of fishes, including 24 species from Tai Lake, a main source of fish in Nanjing market, were examined for PAHs (Wang et al. 2012) (Table 1). Potency equivalence factors (PEFs) Environmental data Drinking water of families in Nanjing (n = 25) was collected in December 2013. The water samples covered five major water treatment plants in Nanjing. The 16 PAHs that have been identified as priority pollutants by the USEPA were examined. Standard target organic constituents were obtained from Supelco (Bellefont, PA, USA). A solid-phase extraction (SPE) method was used to extract the target contaminants in water samples (Shi et al. 2012). Quality assurance analyses were conducted according to previous studies (Hu et al. 2013). Concentration of PAHs in ambient air, soil, and food of Nanjing was available from previous monitoring and investigations, which have been published in the peer-reviewed literature. PAHs in air were quantified by use of a week-long period (n = 28) on a day/night basis in the summer and winter of 2004. Samples were collected by the use of PM2.5 high-volume air samplers (Wang et al. 2007). In 2007, 126 samples of soil were collected from five districts of Nanjing and concentrations of PAHs were determined by the use of high- 123 Information on relative potencies was not available for all of the PAHs. The carcinogenic risk posed by a multicomponent PAHs mixture can be estimated by conversion of the carcinogenic potency of each individual PAH relative to that of B[a]P, which is considered to be the most potent carcinogen among the PAHs. Carcinogenic potency of mixtures of PAHs (B[a]Peq) can be calculated as the sum of the products of B[a]P equivalents (PEF) and concentrations of individual PAHs. This process can simplify and increase accuracy of risk assessments (Chowdhury et al. 2009). There are several sets of PEFs that have been developed by various agencies and scientists. Among these, the PEFs proposed by Nisbet and LaGoy have been demonstrated to be among the most predictive PEFs and have been commonly used (Petry et al. 1996; Wang et al. 2011a, b; Peng et al. 2011) (Table 2). Multi-pathway exposure modeling PAHs exist in various environmental media, such as air, water, and soil, and can enter human body via Environ Geochem Health (2015) 37:587–601 591 Table 2 Potency equivalence factors (PEFs) for individual PAHs relative to B[a]P (Nisbet and Lagoy 1992) Compound PEF Compound PEF Naphthalene (Nap) 0.001 Benz[a]anthracene (BA) 0.1 Acenaphthylene (Ac) 0.001 Chrysene (Chr) 0.01 Acenaphthene (Ace) Fluorene (Flu) 0.001 0.001 Benzo[b]fluoranthene (BbF) Benzo[k]fluoranthene (BkF) 0.1 0.1 Phenanthrene (Phe) 0.001 Benzo[a]pyrene (B[a]P) 1 Anthracene (Ant) 0.01 Indeno[1,2,3-c,d]pyrene (InP) 0.1 Fluoranthene (Flt) 0.001 Dibenz[a,h]anthracene (DBA) 1 Pyrene (Pyr) 0.001 Benzo[g,h,i]perylene (BghiP) 0.01 Fig. 2 Human exposure to PAHs through multipathways different routes, including ingestion, inhalation, or dermal contact (Fig. 2). Assessments of cumulative exposure estimate intensity, frequency, and duration of exposures to agents, such as PAHs present in the environment (NRC 1983b). Time-averaged dose can be linked to the exposure medium concentration and is used here for the exposure analysis [Eq. 1, adapted from (USEPA 2004)]. C IR EF ED ADI ¼ BW AT year); ED is duration of exposure (years); BW is body weight (kg); and AT is duration over which the dose is averaged (days). Contaminants in water and soil can adhere to exposed skin and enter the human body through dermal absorption. Average daily intakes of the PAHs through dermal contact of water can be estimated using Eq. 2. ADIderw ¼ ð1Þ where ADI is the average daily exposure dose (mg/kg/ day); C is the B[a]P equivalent concentration in the exposure medium, expressed as mg/L, mg/kg, or mg/ m3; IR is the rate of ingestion, expressed as L/day, kg/ day, or m3/day; EF is the exposure frequency (days/ Cw Abath Kp t EF ED BW AT ð2Þ where Cw is the concentration of B[a]P equivalents in water, Abath is the total skin surface area (cm2), Kp is the dermal permeability coefficient of the PAHs in water (cm/h), and t is the time for shower (min/day). Average daily intakes of the PAHs through dermal contact of soil can be estimated using Eq. 3. 123 592 ADIders ¼ Environ Geochem Health (2015) 37:587–601 Cs As AF ABS EF ED BW AT ð3Þ where Cs is the B[a]P equivalent concentration in soil, As is the dermal surface area exposed (cm2), AF is the soil-to-skin adherence factor (mg/cm2/day), and ABS is the dermal absorption fraction (dimensionless). Incremental lifetime cancer risk (ILCR) model Risks of additional cancers in adults, children, and infants were evaluated by applying the incremental lifetime cancer risk (ILCR) model (USEPA 2004). The model assumes that exposure to any amount of a carcinogen will increase the risk of cancer, that is, there is no safe or threshold dosage. A slope factor multiplied by the average daily intake gives a maximum probability that a receptor will develop cancer from exposure to a chemical over lifetime (USEPA 2004). And risks are assumed to be additive from multiple chemicals and routes (Eq. 4). Risk ¼ ADI SF ð4Þ where risk is the incremental cancer risk to an individual over a lifetime, which is accumulative across dermal, oral, and inhalation exposure; SF is the carcinogenicity slope factor (mg/kg/day)-1, which represents an upper bound estimate of the probability of individual’s carcinogenic response per unit intake dose of chemical over lifetime. SFs of B[a]P for each exposure pathway are obtained from open databases or the literature and are presented in Table 3. Monte Carlo simulation Uncertainties arising from data scarcity, parameter variability, and model limitations can affect defining, evaluating, and choosing various options for management of risks (Chen et al. 2011; Qu et al. 2012a, b; Chen et al. 2015). Integration of variability and uncertainty into risk estimation can lead to a more Table 3 Carcinogenic slope factors of PAHs utilized in the human health risk calculations SF (mg/kg/day)-1 References Inhalation 3.9 OEHHA (2011) Oral 7.3 USEPA (2012) Dermal 25 Knafla et al. (2006) 123 realistic understanding of risk (Chen and Chen 2012; Chen et al. 2013). Monte Carlo simulation techniques, which are often used to address uncertainty in assessments of risks to humans (Mari et al. 2009), provide quantitative estimates of probabilities of exposure and adverse outcomes (Thompson et al. 1992). Repeated sampling based on probability distributions of exposure variables results in a frequency distribution of risk. Variables used in the simulation include parameters describing demographics of the population, rates of inhalation, ingestion, dermal absorption, and risk factors. In March 2014, a dietary survey was conducted for the general population of Nanjing by the use of a questionnaire given to 127 randomly selected individuals. The rate of ingestion of fish, expressed as grams per day (g/day), was obtained, while values for other parameters were taken from the open published literature (Table 4). Sensitivity analyses were conducted to identify the most significant parameters that were included in the risk estimation. Spearman’s rank order correlation coefficients between inputs and outputs were calculated. The Monte Carlo simulation and sensitivity analysis were performed using @Risk software (version 5.5; Palisade Corporation; Ithaca, NY, USA). To ensure the stability of results, simulations were run for 5,000 iterations with each parameter sampled independently. Concentrations of B[a]Peq were estimated by the multiplication of the individual PAH concentration by its PEF on the basis of the Monte Carlo simulation. The probability density functions were fitted to the observed concentration data values using the @Risk Best Fit function. The goodness of fit was determined using the Chi-squared statistic, the Kolmogorov–Smirnov statistic, and the Anderson–Darling statistic. Results and discussion B[a]P equivalent concentration (B[a]Peq) Lognormal curves were found to be the distributions that best fit the distribution of concentrations of B[a]Peq in various environmental media (Fig. 3). Firstly, the mean concentration of B[a]Peq in air was estimated to be 8.74 ng/m3. It is much lower than that in occupational environments, like in carbon black manufacturing industry in southern Taiwan (308 ng/ Environ Geochem Health (2015) 37:587–601 593 Table 4 Risk parameters used for the Monte Carlo simulation Parameter Symbol Units Infants Children Adults References Age – Year 0–1 2–18 19–70 Body weight BW kg 6.79 ± 1.27 37.3 ± 9.1 58.7 ± 12.0 Chen and Liao (2006), Xiao et al. (2005) IRa m3/day 5.05 ± 0.49 9.67 ± 2.39 12.44 ± 1.27 Wang et al. (2009) Population parameter Inhalation parameter Inhalation rate Ingestion parameter Ingestion rate of water IRw mL/day 283.25 ± 91.48 497.35 ± 138.28 1,366 ± 728 USEPA (1997, 2008) Ingestion rate of soil IRs mg/day 0–30 24 ± 4 25 (0.1–50) Stanek et al. (2001), USEPA (2008), LaGoy (1987) Ingestion rate of fish IRf mg/day 4.16 ± 2.37 27.45 ± 5.52 61.25 ± 13.86 Abath m2 0.39 ± 0.05 1.09 ± 0.37 1.67 ± 0.10 Wang et al. (2008) 719 ± 1.19 860 (430–2,160) 1,530 (760–4,220) Wang et al. (2008), Chen and Liao (2006) 0.04 0.65 ± 1.2 0.49 ± 0.54 Dermal parameter Total skin surface area 2 Exposed skin surface area As cm Soil-to-skin adherence factor Time for shower AF mg/cm2-day t min/day 15 18.41 ± 1.32 10.4 (3–61) USEPA (2004), Finley et al. (1994) USEPA (1997, 2008) Dermal absorption factor ABS Unitless 0.13 0.13 0.13 USEPA (2004) Dermal permeability coefficient Kp cm/h 0.7 0.7 0.7 USEPA (2004) Exposure frequency EF Days/year 345 (180–365) 345 (180–365) 345 (180–365) Smith (1994) Averaging time AT Days 25,550 25,550 25,550 USEPA (1997) Exposure duration ED Year 1 17 52 USEPA (1997, 2008) Risk model parameter The mean and standard deviation were used for lognormal distributions; minimum and maximum for uniform distributions; and mean, minimum, and maximum for triangular distributions m3) and at road intersections in the city of Tianjin (82.4 ng/m3), but higher than that on campus in Tianjin (2.4 ng/m3) (Tsai et al. 2001; Hu et al. 2007). Mean concentration of B[a]Peq in air in Nanjing was close to the current annual limit on concentration of B[a]P in China (10 ng/m3). However, beginning in 2016, the newly recommended air quality standard of B[a]P annual limit will be reduced to 1 ng/m3 (MEP 2012). Secondly, the concentration of B[a]Peq in drinking water in Nanjing was 0.004 ng/L on average and far less than the national standard limit of 10 ng/L (MOH 2006). Thirdly, the mean concentration of B[a]Peq in soil in Nanjing was 8.19 ng/g, which is much less than the proposed national limit value of 100 ng/g, dm for farmland (MEP 2008). Compared to the concentration of B[a]Peq in road dust in polluted industrial areas in Ulsan, Korea (0.93–16.74 lg/g) (Dong and Lee 2009), mean concentration of B[a]Peq in soil in Nanjing was relatively low. Furthermore, this value was also similar to the background concentrations of B[a]P in uncontaminated soils in Poland that ranged from 1.5 to 78 ng/g (Wcisło 1998), but a little higher than that in Tarragona, Spain (\2 ng/g) (Nadal et al. 2004). Finally, contamination by PAHs might occur during processing of food or through environmental pollution, especially in fish. The mean concentration of B[a]Peq in fish used in this study was 4.58 ng/g. This concentration is slightly less than the national limit of B[a]P in aquatic products in China (5 ng/g) (MOH 2012), but exceeded the European limit of B[a]P in muscle meat of fish (2 ng/g) (CEC 2006). Compared to previous studies, B[a]Peq in fish in Nanjing was less than that in Taiyuan (5.71 ng/g) (Xia et al. 2010), but greater than that in Tianjin, northern 123 594 Environ Geochem Health (2015) 37:587–601 Fig. 3 B[a]Peq concentrations of each environmental media China (1.52 ng/g), and Seoul, South Korea (0.15–0.52 ng/g) (Li 2007; Yoon et al. 2007). The relative proportions of B[a]Peq varied among matrices and media. The concentration of B[a]Peq in air was primarily contributed by B[a]P and B[b]F, which accounted for 46 and 30 % of the total PAHs, respectively. Anthracene contributed the greatest proportion (71 %) to the concentration of B[a]Peq in water, followed by Acenaphthene and Fluorene with contributions of 8.3 and 7.9 %, respectively. In soil, DBA, B[a]P, and B[b]F were the predominant contributors to the concentration of B[a]Peq, providing 41, 24, and 21 %, respectively. In fish, B[a]P, BA, and Anthracene contributed 36, 14, and 12 %, respectively, to the B[a]Peq. For infants, inhalation and ingestion of fish were the dominant pathways of exposure, whose median B[a]Peq exposure doses were 8.25 9 10-8 and 3.02 9 10-8 mg/kg, bm/day, respectively. The B[a]Peq daily exposure dose from other pathways ranged from 2.03 9 10-12 to 2.17 9 10-10 mg/kg, bm/day. In children and adults, dose of B[a]Peq was greater than that of infants. Moreover, ingestion of fish was the main pathway of exposure with B[a]Peq mean daily exposure doses of 7.04 9 10-7 and 2.91 9 10-6 mg/kg, bw/day for children and adults, respectively. Following ingestion of fish, inhalation exposure doses were 4.71 9 10-7 and 1.43 9 10-6 mg/kg, bm/day, respectively. Similarly, the contribution from other exposure pathways was relatively slight. Analysis of exposure Estimation of risk Probability functions of concentrations of B[a]Peq in air, water, soil, and fish were used in Monte Carlo simulations to estimate exposure to B[a]Peq (Fig. 4). From daily exposures via different exposure pathways, the incremental lifetime cancer risks were estimated using Eq. 4, and Monte Carlo simulation 123 B[a]Peq daily exposure doses of adults (mg kg-1 d-1) B[a]Peq daily exposure doses of children (mg kg-1 d-1) B[a]Peq daily exposure doses of infants (mg kg-1 d-1) Environ Geochem Health (2015) 37:587–601 595 95th 75th mean 25th 5th 1.4E-07 1.2E-07 6.0E-10 1.0E-07 5.0E-10 4.0E-10 8.0E-08 3.0E-10 6.0E-08 2.0E-10 1.0E-10 4.0E-08 1.0E-12 Water ingestion Soil ingestion Dermal contact Dermal contact of water of soil Water ingestion Soil ingestion Dermal contact Dermal contact of water of soil 2.0E-08 0.0E+00 Inhalation Fish ingestion 1.6E-06 1.4E-06 1.2E-08 1.2E-06 1.0E-08 1.0E-06 8.0E-09 8.0E-07 6.0E-09 4.0E-09 6.0E-07 2.0E-09 4.0E-07 0.0E+00 2.0E-07 0.0E+00 Inhalation Fish ingestion 7.0E-06 6.0E-06 4.0E-08 5.0E-06 3.0E-08 4.0E-06 2.0E-08 3.0E-06 1.0E-08 2.0E-06 0.0E+00 Water ingestion Soil ingestion Dermal contact Dermal contact of water of soil 1.0E-06 0.0E+00 Inhalation Fish ingestion Fig. 4 Box and whisker plots of B[a]Peq daily exposure doses for three age groups 123 596 Fig. 5 Total incremental lifetime cancer risk to different age groups was used to calculate cumulative probability of the total risks of different age groups (Fig. 5). Overall, the incremental lifetime cancer risks posed by exposure to PAHs through different exposure pathways were in the order of adults [ children [ infants. The total risk to adults was 2.62 9 10-5 on average (i.e., 26 in a million). For children, the mean risk was 7.08 9 10-6, which was much less than that of the adults. Because of physiological and behavioral differences, the exposures of infants were expected to differ from those of adults and children. The average risk to infants for that portion of their lives was estimated to 5.31 9 10-7, which was less than those of adults and children. There are no absolute criteria for acceptable number of additional cancers over a lifetime. In most jurisdictions, the one-in-one-million (1 9 10-6) chance of additional cancers proposed by the USEPA is frequently used as a management goal for risks posed by environmental contamination. However, risks ranging from 1-in-10,000 (1 9 10-4) to 1-in1,000,000 (1 9 10-6) are generally considered acceptable, depending on the situation and circumstances of exposure (HC 2004). Compared with this risk range, the total risks combined from different exposure pathways of infants (5.31 9 10-7 on average) were minimal. For children, the mean carcinogenic risk was 7.08 9 10-6, and the probabilities of total risks exceeding 1 9 10-5 and 1 9 10-4 were 15 % and 0, respectively. For adults, the carcinogenic risks were not significant from the mean obtained based on point estimations (2.62 9 10-5). However, 123 Environ Geochem Health (2015) 37:587–601 from the cumulative probability curve, it was found that probabilities of total risks to adults exceeding 1 9 10-5 and 1 9 10-4 were as high as 97.6 and 0.5 %, respectively. That is, there was a potential carcinogenic risk to local residents. Thus, it is unilateral to judge the risk only by mean values or point estimation. Probabilistic risk assessments, based on Monte Carlo simulation, can provide more comprehensive information, which is crucially important for decision making of risk control. Multi-pathway assessment of exposure was conducted to determine relative contributions of each exposure pathway (Table 5). For all age groups, the incremental lifetime risks of additional cancers posed by ingestion of fish and inhalation were the greatest and together accounted for nearly 99 % of the total risks. For adults, on average, ingestion of fish and inhalation contributed 81.81 and 17.07 %, respectively, to the risk of additional cancers. Other pathways of exposure contributed only 1 %. Ingestion of fish and inhalation contributed 72.77 and 25.97 %, respectively, to the total risk to children. For infants, inhalation contributed most to the total risk, accounting for 57.56 %, whereas ingestion of fish contributed 41.86 %. The contribution of inhalation reflects the increasingly grim situation of air pollution control in the studied area, which is a microcosm of the conditions prevalent in China. The source of fish in this study was Tai Lake, a eutrophic shallow lake that is surrounded by large industrial areas. The contribution of ingestion of fish to the overall risk shows that food ingestion is the overwhelmingly dominant exposure pathway of environmental PAHs. Therefore, assessments of risks to health of humans should be conducted further for aquaculture and fisheries activities in Tai Lake to avoid health risk through aquatic products consumption. Because of the small concentration of B[a]Peq in drinking water, the ILCRs from ingestion and dermal contact of water were essentially negligible. With regard to the ingestion and dermal contact of soil, they contributed little to the total risks and could be considered negligible for all three age groups. For substances deemed to be carcinogenic, the estimated exposure is multiplied by the appropriate slope factor to derive an estimate of the potential risk associated with that exposure. However, this model does not assume any threshold for effects. As such, it is 2.88E-07 2.70E-07 8.40E-07 8.88E-08 8.45E-07 6.90E-10 Dermal contact of soil 2.28E-09 1.35E-09 4.97E-10 8.81E-09 2.61E-07 3.71E-08 6.12E-09 1.35E-05 2.13E-05 5.39E-09 1.69E-08 4.63E-05 8.20E-06 8.00E-10 3.40E-06 1.29E-09 5.15E-06 1.33E-09 1.15E-05 3.84E-09 1.93E-06 3.10E-10 1.14E-10 1.72E-07 2.22E-07 6.10E-08 3.91E-11 Ingestion of fish Dermal contact of water 5.35E-07 1.27E-10 3.53E-09 8.04E-09 3.65E-09 1.14E-09 1.59E-09 3.72E-09 1.41 E-10 Ingestion of soil 3.50E-10 3.69E-10 8.93E-09 1.57E-08 3.24E-08 1.35E-06 1.47E-08 4.00E-09 3.85E-10 4.45E-06 6.90E-06 1.11E-09 9.01E-11 8.13E-11 8.57E-11 2.51E-06 7.70E-07 1.84E-06 2.49E-10 2.20E-11 1.42E-11 9.34E-8 3.05E-07 1.47E-11 4.10E-11 3.80E-12 Ingestion of water 4.77E-07 1.74E-07 Inhalation Mean 95 % 8.70E-07 3.28E-06 5% SD Mean 95 % 5% 5% SD Children Infants Exposure pathway Table 5 Incremental lifetime cancer risks of different exposure pathways Adults 95 % Mean SD Environ Geochem Health (2015) 37:587–601 597 believed (but not proved) that the slope factor for carcinogenic substances will overestimate the actual cancer incidence associated with low-dose exposure to environmental pollutants (Kelly and Cardon 1991). Furthermore, the B[a]Peq-based approach is necessarily limited to 16 priority PAHs and does not account for the toxicity of all PAHs to which the population is generally exposed. It should also be pointed out that this study presented modeled estimates of exposure and modeled estimates of risk, but no evidence of actual health effects was reported. More detailed and in-depth health investigation is necessary in the future to examine whether adverse health outcomes occur. Despite these limitations, this study has the merit of taking into account various exposure pathways and estimating the potential risk from a probabilistic view by applying Monte Carlo simulation approach to show the actual PAHs profiles encountered in environmental settings. Sensitivity analysis Uncertainties are inherent in quantitative risk assessment. For this reason, a quantitative sensitivity analysis was conducted to further evaluate the variability and uncertainty of the parameters that contributed most significantly to the risk estimations. The results of the sensitivity analysis for each exposure pathway in the assessment model were shown in the form of tornado plots illustrating the Spearman’s rank order correlation coefficients (Fig. 6). The tornado plot gave both the magnitude and direction (positive or negative) of the correlation. The results of the analysis indicated that the concentration of B[a]Peq in fish (Cf), the body weight (BW), the ingestion rate of fish (IRf), the exposure frequency (EF), and the B[a]Peq concentration in air (Ca) were the most influential variables for all the three age groups. Therefore, in order to increase accuracy of the risk estimation, efforts should focus on more accurately and precisely defining the probability distributions of the Cf, BW, IRf, EF, and Ca parameters. For instance, more survey and examination of PAHs in different environmental media, especially in meat, rice, vegetable, and air, is needed. It is also necessary to conduct more surveys of diets of local people and their cooking habits to illustrate the importance of cooking, which might influence both exposure parameters and concentration of PAHs in food. 123 598 Fig. 6 Sensitivity analysis for incremental lifetime cancer risk models for three age groups 123 Environ Geochem Health (2015) 37:587–601 Environ Geochem Health (2015) 37:587–601 Conclusions Aggregate probabilistic estimates of exposures of humans to PAHs via various environmental media and pathways were developed, and a multi-pathway framework for assessment of risks was proposed by integrating PAHs potency equivalence factors, risk estimation modeling, and the Monte Carlo simulations. In the case study of Nanjing, People’s Republic of China, incremental lifetime risks of additional cancers posed by 16 priority PAHs in air, water, soil, and fish through multiple routes of exposure, including oral ingestion, dermal absorption, and inhalation exposure, were estimated for infants, children, and adults. Concentrations of PAHs in the environment of Nanjing represented a risk of 2.62 9 10-5 for additional cancers in adults exposed via all pathways. Exposures to B[a]P, B[b]F, and BA through ingestion of fish and inhalation contributed 99 % to the total risks. Thus, the potential health hazard from the PAHs deserves more attention in the future. Results of this study imply that multipathway health risk assessment might be a useful tool to estimate the health risk posed by environmental PAHs pollution. Through identification of key exposure pathways and the selection of groups at greater risk, more practical control of pollution and thus more effective measure to alleviate the risk can be formulated to ensure the most effective and least costly methods of minimizing risks of additional cancers to the public. It may be helpful to the understanding of PAHs-triggered environmental health problem and further benefit the pollution control and risk alleviation for the counties and regions those troubled with PAHs pollution. However, this study still has uncertainties and limitations. Importantly, because of the absence of a local dietary survey and of information concerning the PAH concentrations in the local foods, only fish was included in this study. More detailed information on the PAHs concentrations of different media is required to reduce the uncertainties and limit the variabilities. Moreover, on the basis of sensitivity analysis results, further research should be directed to better characterizing those parameters that could most effectively improve the estimation results. Acknowledgments This work was supported by National Natural Science Foundation (Grant No. 41201545), National Meta-Program for Science and Technology of Water Pollution Control (2012ZX07506-001), National 863 Project (2013AA 06A309), and Jiangsu Science Program for Environmental 599 Protection (2014038). Prof. Giesy was supported by the program of 2012 ‘‘High Level Foreign Experts’’ (#GDW20123200120) funded by the State Administration of Foreign Experts Affairs, the P.R. China to Nanjing University and the Einstein Professor Program of the Chinese Academy of Sciences. He was also supported by the Canada Research Chair program, a Visiting Distinguished Professorship in the Department of Biology and Chemistry and State Key Laboratory in Marine Pollution, City University of Hong Kong. References Binet, S., Pfohl-Leszkowicz, A., Brandt, H., Lafontaine, M., & Castegnaro, M. (2002). Bitumen fumes: Review of work on the potential risk to workers and the present knowledge on its origin. The Science of the Total Environment, 300(1–3), 37–49. Cao, Z., Liu, J., Li, Y., & Ma, M. (2010). Distribution and source apportionment of polycyclic aromatic hydrocarbons (PAH) in water and sediments of the Luan River, China. Toxicological & Environ Chemistry, 92(4), 707–720. CEC. (2006). Commission regulation (EC) No 1881/2006: Maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, 1, 5–24. Chen, S., & Chen, B. (2012). Defining indirect uncertainty in system-based risk management. Ecological Informatics, 10, 10–16. Chen, S. C., & Liao, C. M. (2006). Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Science of the Total Environment, 366(1), 112–123. Chen, S., Chen, B., & Fath, B. D. (2013). Ecological risk assessment on the system scale: A review of state-of-theart models and future perspectives. Ecological Modelling, 250, 25–33. Chen, S., Chen, B., & Fath, B. D. (2015). Assessing the cumulative environmental impact of hydropower construction on river systems based on energy network model. Renewable and Sustainable Energy Reviews, 42, 78–92. Chen, S., Fath, B. D., & Chen, B. (2011). Information-based network environ analysis: A system perspective for ecological risk assessment. Ecological Indicators, 11(6), 1664–1672. Chowdhury, K. H., Husain, T., Veitch, B., & Hawboldt, K. (2009). Probabilistic risk assessment of polycyclic aromatic hydrocarbons (PAHs) in produced water. Human and Ecological Risk Assessment, 15(5), 1049–1063. Dong, T. T., & Lee, B. K. (2009). Characteristics, toxicity, and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in road dust of Ulsan, Korea. Chemosphere, 74, 1245–1253. Feng, C., Luo, Q., & Wang, Z. (2009). Concentration levels and potential ecological risks of polycyclic aromatic hydrocarbons in Chinese rivers. Water Quality, Exposure and Health, 1(2), 105–113. Finley, B. L., Scott, P. K., & Mighthall, D. A. (1994). Development of a standard soil-to-skin adherence probability density-function for use in Monte-Carlo analyses of dermal exposure. Risk Analysis, 14(4), 555–569. 123 600 Health Canada (HC). (2004). Federal contaminated site risk assessment in Canada part I: Guidance on human health preliminary quantitative risk assessment (PQRA). Ottawa: Health Canada (HC). Hu, Y. D., Bai, Z. P., Zhang, L. W., Wang, X., Zhang, L., et al. (2007). Health risk assessment for traffic policemen exposed to polycyclic aromatic hydrocarbons (PAHs) in Tianjin, China. Science of the Total Environment, 382(2–3), 240–250. Hu, X., Shi, W., Wei, S., Zhang, X., Feng, J., Hu, G., et al. (2013). Occurrence and potential causes of androgenic activities in source and drinking water in China. Environmental Science and Technology, 47(18), 10591–10600. Integrated Risk Information System. (2012). http://www.epa. gov/IRIS/ Ji, G., Gu, A., Zhou, Y., Shi, X., Xia, Y., Long, Y., et al. (2010). Interactions between exposure to environmental polycyclic aromatic hydrocarbons and DNA repair gene polymorphisms on bulky DNA adducts in human sperm. PLoS One, 5(10), e13145. Kelly, K. E., & Cardon, N. (1991). The myth of 10-6 as a definition of acceptable risk. In 84th Annual meeting and exhibition of the air and waste management association, Vancouver, BC, pp. 16–21. Knafla, A., Phillipps, K., Brecher, R., Petrovic, S., & Richardson, M. (2006). Development of a dermal cancer slope factor for benzo[a]pyrene. Regulatory Toxicology and Pharmacology, 45(2), 159–168. LaGoy, P. (1987). Estimated soil ingestion rates for use in risk assessment. Risk Analysis, 7, 355–359. Li, X. (2007). Spatial distribution pattern of emission, dispersion and exposure of polycyclic aromatic hydrocarbons in Tianjin, China. Beijing: Peking UniversityChina. Lin, T., Qin, Y., Zheng, B., Li, Y., Zhang, L., & Guo, Z. (2012). Sedimentary record of polycyclic aromatic hydrocarbons in a reservoir in Northeast China. Environmental Pollution, 163, 256–260. Mari, M., Nadal, M., Schuhmacher, M., & Domingo, J. L. (2009). Exposure to heavy metals and PCDD/Fs by the population living in the vicinity of a hazardous waste landfill in Catalonia, Spain: Health risk assessment. Environment International, 35(7), 1034–1039. Marin, C., Guvanasen, V., & Saleem, Z. (2003). The 3MRA risk assessment framework—A flexible approach for performing multimedia, multipathway, and multireceptor risk assessments under uncertainty. Human and Ecological Risk Assessment, 9(7), 1655–1677. MEP. (2008). Environmental quality standards for soils (draft). Beijing: Ministry of Environmental Protection of China. MEP. (2012). Ambient air quality standards of China (GB 3095-2012). Beijing: Ministry of Environmental Protection of China. MOH. (2006). Standards for drinking water quality of China (GB5749-2006). Beijing: Ministry of Health of China. MOH. (2012). Limits for contaminants in food (GB2762-2012). Beijing: Ministry of Health of China. Nadal, M., Schuhmacher, M., & Domingo, J. L. (2004). Levels of PAHs in soil and vegetation samples from Tarragona County, Spain. Environmental Pollution, 132(1), 1–11. National Research Council (NRC). (1983). Risk assessment in federal government: Managing the process. Washington, DC: National Academy Press. 123 Environ Geochem Health (2015) 37:587–601 National Research Council NRC. (1983). Risk assessment in the federal government: managing the process (National Research Council ed.). Washington, DC: National Academy Press. Nisbet, I. C. T., & Lagoy, P. K. (1992). Toxic equivalency factors (TEFs) for polycyclic aromatic-hydrocarbons (PAHs). Regulatory Toxicology and Pharmacology, 16(3), 290–300. Orecchio, S., & Papuzza, V. (2009). Levels, fingerprint and daily intake of polycyclic aromatic hydrocarbons (PAHs) in bread baked using wood as fuel. Journal of Hazardous Materials, 164(2–3), 876–883. Peng, C., Chen, W., Liao, X., Wang, M., Ouyang, Z., Jiao, W., et al. (2011). Polycyclic aromatic hydrocarbons in urban soils of Beijing: Status, sources, distribution and potential risk. Environmental Pollution, 159(3), 802–808. Petry, T., Schmid, P., & Schlatter, C. (1996). The use of toxic equivalency factors in assessing occupational and environmental health risk associated with exposure to airborne mixtures of polycyclic aromatic hydrocarbons (PAHs). Chemosphere, 32(4), 639–648. Qu, C. S., Ma, Z.-W., Yang, J., Liu, Y., Bi, J., & Huang, L. (2012a). Human exposure pathways of heavy metals in a lead–zinc mining area, Jiangsu Province, China. PLoS ONE, 7(11), e46793. Qu, C. S., Sun, K., Wang, S. R., Huang, L., & Bi, J. (2012b). Monte Carlo simulation-based health risk assessment of heavy metal soil pollution—A case study in the Qixia mining area, China. Human and Ecological Risk Assessment, 18(4), 1–18. Shi, W., Hu, X., Zhang, F., Hu, G., Hao, Y., Zhang, X., et al. (2012). Occurrence of thyroid hormone activities in drinking water from eastern China: Contributions of phthalate esters. Environmental Science and Technology, 46(3), 1811–1818. Shi, W., Zhang, F., Zhang, X., Su, G., Wei, S., Liu, H., et al. (2011). Identification of trace organic pollutants in freshwater sources in Eastern China and estimation of their associated human health risks. Ecotoxicology, 20(5), 1099–1106. Smith, R. (1994). Use of Monte Carlo simulation for human exposure assessment at a superfund site. Risk Analysis, 14, 433–439. Srogi, K. (2007). Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environmental Chemistry Letters, 5(4), 169–195. Stanek, E. J., Calabrese, E. J., & Zorn, M. (2001). Soil ingestion distributions for Monte Carlo risk assessment in children. Human and Ecological Risk Assessment, 7(2), 357–368. Sun, F., Littlejohn, D., & David Gibson, M. (1998). Ultrasonication extraction and solid phase extraction clean-up for determination of US EPA 16 priority pollutant polycyclic aromatic hydrocarbons in soils by reversed-phase liquid chromatography with ultraviolet absorption detection. Analytica Chimica Acta, 364(1–3), 1–11. Thompson, K., Burmaster, D., & Crouch, E. (1992). Monte Carlo techniques for quantitative uncertainty analysis in public health risk assessments. Risk Analysis, 12(1), 53–63. Toxicity Criteria Database. (2011). California Office of Environmental Health Hazard Assessment. http://www.oehha. ca.gov/tcdb/ Environ Geochem Health (2015) 37:587–601 Tsai, P., Shieh, H. Y., Lee, W. J., & Lai, S. O. (2001). Healthrisk assessment for workers exposed to polycyclic aromatic hydrocarbons (PAHs) in a carbon black manufacturing industry. Science of the Total Environment, 278(1–3), 137–150. USEPA. (1997). Exposure factors handbook (final report) 1997. Washington, DC: USEPA. USEPA. (2004). Risk assessment guidance for superfund volume I: Human health evaluation manual (Part E, Supplemental Guidance for Dermal Risk Assessment). Washington, DC: USEPA. USEPA. (2008). Child-specific exposure factors handbook. Washington, DC: USEPA. Wang, W., Huang, M. J., Kang, Y., Wang, H. S., Leung, O. W., et al. (2011a). Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: Status, sources and human health risk assessment. Science of the Total Environment, 409(21), 4519–4527. Wang, G., Kawamura, K., Zhao, X., Li, Q., Dai, Z., & Niu, H. (2007). Identification, abundance and seasonal variation of anthropogenic organic aerosols from a mega-city in China. Atmospheric Environment, 41(2), 407–416. Wang, Z., Liu, S. Q., Chen, X. M., & Lin, C. Y. (2008). Estimates of the exposed dermal surface area of Chinese in view of human health risk assessment. Journal of Safety and Environment, 8(4), 152–156. Wang, W., Simonich, S., Giri, B., Chang, Y., Zhang, Y., Jia, Y., et al. (2011b). Atmospheric concentrations and air-soil gas exchange of polycyclic aromatic hydrocarbons (PAHs) in remote, rural village and urban areas of Beijing–Tianjin region, North China. Science of the Total Environment, 409(15), 2942–2950. Wang, Z. S., Wu, T., Duan, X. L., Wang, S., & Zhang, W. J. (2009). Research on inhalation rate exposure factors of Chinese residents in environmental health risk assessment. Research of Environmental Sciences, 22(10), 1171–1175. Wang, D.-Q., Yu, Y.-X., Zhang, X.-Y., Zhang, S.-H., Pang, Y.P., Zhang, X.-L., et al. (2012). Polycyclic aromatic hydrocarbons and organochlorine pesticides in fish from Taihu Lake: Their levels, sources, and biomagnification. Ecotoxicology and Environmental Safety, 82, 63–70. Wcisło, E. (1998). Soil contamination with polycyclic aromatic hydrocarbons (PAHs) in Poland—A review. Polish Journal of Environmental Studies, 7(5), 267–272. 601 Xia, Z., Duan, X., Qiu, W., Liu, D., Wang, B., Tao, S., et al. (2010). Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Science of the Total Environment, 408(22), 5331–5337. Xiao, G., Li, Y., & Ma, G. (2005). Study on weight and height of the Chinese people and the differences between 1992 and 2002. Chinese Journal of Epidemiology, 26(7), 489–493. Xu, S., Liu, W., & Tao, S. (2006). Emission of polycyclic aromatic hydrocarbons in China. Environmental Science and Technology, 40(3), 702–708. Yin, C. Q., Jiang, X., Yang, X. L., Bian, Y. R., & Wang, F. (2008). Polycyclic aromatic hydrocarbons in soils in the vicinity of Nanjing, China. Chemosphere, 73(3), 389–394. Yoon, E., Park, K., & Lee, H. (2007). Estimation of excess cancer risk on time-weighted lifetime average daily intake of PAHs from food ingestion. Human and Ecological Risk Assessment, 13(3), 669–680. Zhang, J., Qu, C., Qi, S., Cao, J., Zhan, C., Xing, X., et al. (2014). Polycyclic aromatic hydrocarbons (PAHs) in atmospheric dustfall from the industrial corridor in Hubei Province, Central China. Environmental Geochemistry and Health. doi:10.1007/s10653-014-9647-y. Zhang, Y., Shen, H., Tao, S., & Ma, J. (2011). Modeling the atmospheric transport and outflow of polycyclic aromatic hydrocarbons emitted from China. Atmospheric Environment, 45(17), 2820–2827. Zhang, Y. X., & Tao, S. (2009). Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmospheric Environment, 43, 812–819. Zhang, Y. X., Tao, S., Cao, J., & Coveney, R. M. (2007). Emission of polycyclic aromatic hydrocarbons in China by county. Environmental Science and Technology, 41(3), 683–687. Zhang, Y. X., Tao, S., Shen, H. Z., & Ma, J. M. (2009). Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proceedings of the National Academy of Sciences, 106(50), 21063–21067. 123