E = hv
advertisement

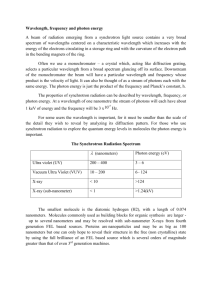
Name ________________________ C= λv E = hv E is the energy of the light in Joules (J), h is a constant which is 6.626 X 10-34 J·s, and v is the frequency of the light in s-1 or waves/s (also called Hertz (Hz). C is the speed of light. C = 3.00 X 108 meters/sec. λ is the wavelength (There are 1 X109 nanometers in one meter.) Use the formulas above and the table below to answer the questions which follow. Frequency, s-1 or (1/s) Color 7.1 X 1014 6.4 X 1014 5.7 X 1014 5.2 X 1014 4.8 X 1014 4.3 X 1014 violet blue yellow orange red green 1. A photon of light with an energy of 2.2 X 10-19 Joules is emitted. What is the frequency of this photon? What color is it? 2. What is the wavelength, in nanometers, of light with a frequency of 7.1 X 1014 Hz? 3. A photon of light has a wavelength of 698 nm. How much energy does it have in Joules? What color is it? 4. How much energy does a photon of light carry if it has a wavelength of 526 nanometers? What color is that photon? 5. What is the wavelength, in nanometers, of a photon of light if it has an energy of 4.01 X 10-19 Joules? What color is it? 6. What is the wavelength, in nanometers, of a photon of light with an energy of 1.66 X 10-19 Joules? What color is it? Answers: 1. 3.3 X 1014 Hz, infrared 2. 420 nm, violet 3. 2.85 X 10-19 J, red 4. 3.78 X 10-19 J, green 5. 496 nm blue/green 6. 1.20 X 103 nm, infrared