Light Powerpoint
advertisement

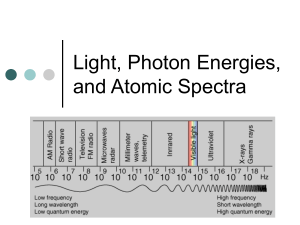
Light, Photon Energies, and Atomic Spectra Questions this answers… Why does FM sound better than AM radio waves? Why am I able to tune in to AM radio stations hundreds of miles away, when FM stations fade in 30-50 miles (or sometimes less)? Why is the sky blue? Is “blue-ray” really better? Why do they put that thing on me when I get an x-ray? Electromagnetic Radiation Electromagnetic radiation (radiant energy) is characterized by its: wavelength (color): (Greek letter lambda) frequency (energy): (Greek letter nu) Electromagnetic Radiation They are related by the equation: c where c = 3.00 x 108 m/s (the speed of light in a vacuum) Waves Wavelength = distance between successive “crests” Frequency = the # of crests passing a given point per second Example: The frequency of violet light is 7.31 x 1014 Hz, and that of red light is 4.57 x 1014 Hz. Calculate the wavelength of each color. 1 Hz = sec-1 or 1/sec Violet: 7.311014 Hz 3.00 10 8 m s 8 m 3.00 10 s 14 1 7.3110 s 4.10 10 m 7 c Example: The frequency of violet light is 7.31 x 1014 Hz, and that of red light is 4.57 x 1014 Hz. Calculate the wavelength of each color. 1 Hz = sec-1 or 1/sec Red: 4.57 10 Hz 14 3.00 10 8 m s 8 m 3.00 10 s 4.57 1014 1s 6.56 10 m 7 c Light When sunlight or white light is passed through a prism, it gives the continuous spectrum observed in a rainbow. We can describe light as composed of particles, or PHOTONS. Each photon of light has a particular amount of energy (a quantum). The amt. of energy possessed by a photon depends on the color of the light. Max Planck (1858-1947) In 1899 a German physicist named Max Planck solved the problem by making a daring assumption. Energy comes in chunks of some minimum size. Planck gave the name “Quantum” (meaning fixed amount) to the smallest quantity of energy Photon Energy The energy of a photon (single quanta) is given by this equation: E h where h = 6.6262 x 10-34 J•s ν = frequency (Hz) Plank was awarded the 1918 Noble Prize. Example: Calculate the energy, in joules, of an individual photon of violet and red light. violet 7.3110 Hz 14 E h Violet: E (6.6262 10 J s)(7.3110 Hz ) -34 14 E (6.6262 10 J s)(7.3110 -34 E 4.84 10 19 J 14 1 s ) Example: Calculate the energy, in joules, of an individual photon of violet and red light. red 4.57 10 Hz 14 E h Red: E (6.6262 10-34 J s)( 4.57 1014 Hz ) E (6.6262 10 J s )( 4.57 10 19 E 3.03 10 J -34 14 1 s ) What does this have to do with electron arrangement in atoms? When all electrons are in the lowest possible energy levels, an atom is said to be in its GROUND STATE. When an atom absorbs energy so that its electrons are “boosted” to higher energy levels, the atom is said to be in an EXCITED STATE. Bright Line Emission Spectrum The light emitted by an element when its electrons return to a lower energy state can be viewed as a bright line emission spectrum. Absorption Spectrum The light absorbed by an element when white light is passed through a sample is illustrated by the absorption spectrum. Note: The wavelengths of light that are absorbed by the gas show up as black lines, and are equal to the wavelengths of light given off in the emission spectrum. Why? Absence of color appears black. Only reflected colors of light are visible. Bright Line Spectra Each element & compound emits a characteristic and unique set of wavelengths. As the temperature of the gas changes, its spectral lines change in intensity; some lines fade out, some appear. The pattern for each substance is the same as for dark line spectra. Note the same pattern for a given element. Light Electronic energy is quantized (only certain values of electron energy are possible). When an electron moves from a lower energy level to a higher energy level in an atom, energy of a characteristic frequency (wavelength) is absorbed. When an electron falls from a higher energy level back to the lower energy level, then radiation of the same frequency (wavelength) is emitted. The bright-line emission spectrum is unique to each element, just like a fingerprint is unique to each person. Example: A green line of wavelength 486 nm is observed in the emission spectrum of hydrogen. Calculate the energy of one photon of this green light. green 486 10 m 9 Green: E hc E (6.6262 10 E 4.09 10 19 -34 c J E h 8 m s )(3.00 10 s ) -9 486 10 m J Example: The green light associated with the aurora borealis is emitted by excited (high-energy) oxygen atoms at 557.7 nm. What is the frequency of this light? 557.7 10 m 9 Green: 3.00 10 557.7 10 m 8 m s -9 14 5.38 10 Hz c