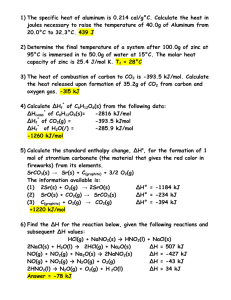
Sample Problem (moles ↔ mass)
advertisement
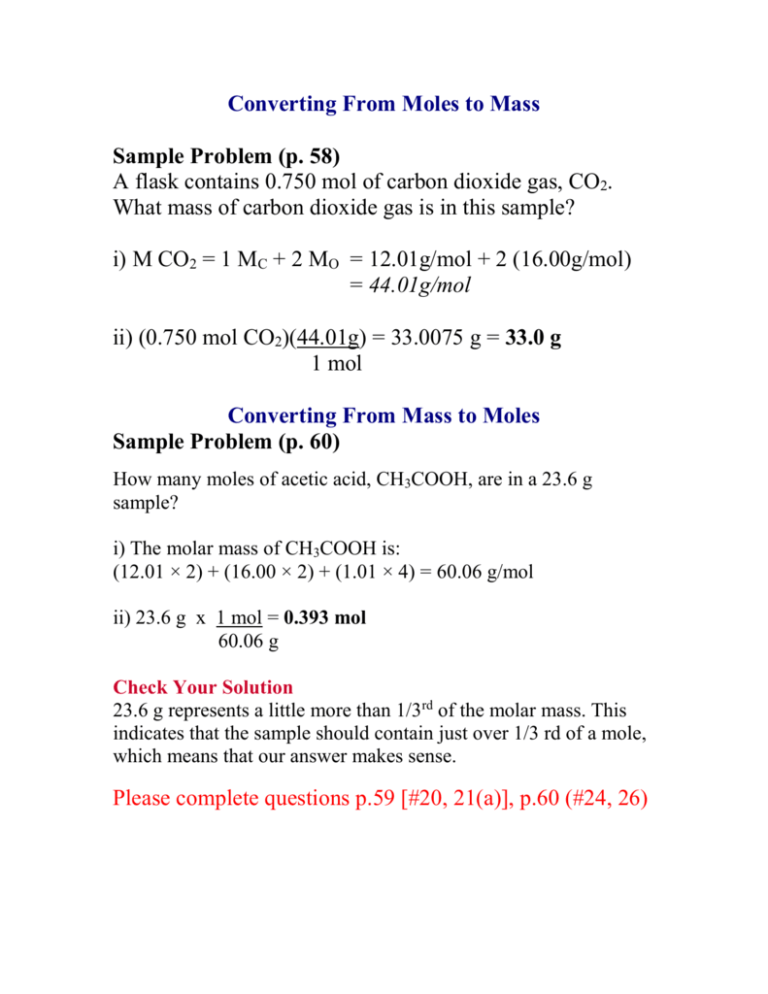
Converting From Moles to Mass Sample Problem (p. 58) A flask contains 0.750 mol of carbon dioxide gas, CO2. What mass of carbon dioxide gas is in this sample? i) M CO2 = 1 MC + 2 MO = 12.01g/mol + 2 (16.00g/mol) = 44.01g/mol ii) (0.750 mol CO2)(44.01g) = 33.0075 g = 33.0 g 1 mol Converting From Mass to Moles Sample Problem (p. 60) How many moles of acetic acid, CH3COOH, are in a 23.6 g sample? i) The molar mass of CH3COOH is: (12.01 × 2) + (16.00 × 2) + (1.01 × 4) = 60.06 g/mol ii) 23.6 g x 1 mol = 0.393 mol 60.06 g Check Your Solution 23.6 g represents a little more than 1/3rd of the molar mass. This indicates that the sample should contain just over 1/3 rd of a mole, which means that our answer makes sense. Please complete questions p.59 [#20, 21(a)], p.60 (#24, 26)