distribution of orexin containing neurones and fibres in the rat brain
advertisement

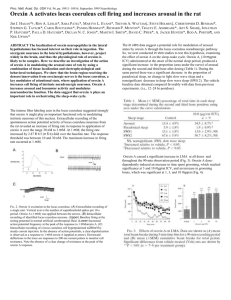
ISRAEL JOURNAL OF VETERINARY MEDICINE Vol. 56 (4) 2001 DISTRIBUTION OF OREXIN-A- AND OREXIN-BCONTAINING NEURONES AND FIBRES IN THE RAT BRAIN AND SPINAL CORD Summary Orexin-A and orexin–B are recently identified neuropeptides that are localized exclusively to the lateral hypothalamic area, the function of which is stimulate feeding. In this study, we employed immunohistochemistry to map the distribution of neurones and fibres containing orexin-A and orexinB in fifteen adult rat brains and spinal cords. Orexin-A and orexin-B cell bodies were concentrated in the lateral hypothalamic and perifornical nuclei and throughout the spinal cord. Orexin-A- and orexinB-immunoreactive fibres were observed extensively in the hypothalamus and spinal cord. The results indicate that orexin-A and -B-immunoreactive neurones contribute to the control of energy homeostasis. The widespread projections of orexin-A and -B fibres within the hypothalamus and other feeding-related centres outside of the hypothalamus, suggests that orexin-A and orexin-B may have an important role in the regulation of feeding. Key Words: Orexin-A, orexin-B, peptide, hypothalamus, feeding. Introduction The hypothalamus represents an important tissue involved in the regulation of nutritional status via the coordination of many neurotransmitter systems implicated in food intake and energy expenditure (1-4). Orexin-A and Orexin-B are, respectively, 33- and 28-amino acid residue peptides, identified from rat brain extracts that activated a G-protein-coupled ‘orphan’ receptor, i.e. that had no known ligand (1). These peptides were independently identified and termed hypocretin-1 and hypocretin-2, although the sequence deduced for the orexins (1). Immunocytochemical studies using antisera against the synthetic fragment orexin-A (14-33) showed that orexin-positive neurones are located in lateral hypothalamic area (LHA) (1). In another study, the antibodies, which were against the synthetic C-terminal sequence of the prepro-hypocretin peptide, labelled a population of neurones with a similar pattern of distribution (5). Immunocytochemical observations on the location of orexin fibres have described the presence of orexin-A and orexin-B immunoreactive processes in the LHA, arcuate, and perifornical nuclei (PeF) and various sites beyond the hypothalamus, such as the thalamic paraventriculer and reuniens nuclei, locus coeruleus, central grey, nucleus of the solitary tract, raphe nuclei and septal nuclei (5-7). The widespread distribution of orexin fibres suggests that the orexins may also be implicated in functions other than feeding and further raises the possibility that these peptides have extensive influences on many target sites in the central nervous system (CNS). The present study was undertaken to characterise the pattern of the distribution of the orexin-A and orexin-B immunoreactivities in the rat brain using immunohistochemistry. Furthermore, we also examined the distribution of orexin fibres in the spinal cord. Materials and Methods Wistar rats of both sexes (200-250 g) were group housed in standard cages and maintained on a 12 h light/dark cycle, 55% relative humidity, with food and water ad libitum. Polyclonal anti-orexin-A and anti-orexin-B antisera were used for the immunohistochemical experiments were raised in New Zealand white rabbits against synthetic peptide C-terminal sequences. All antisera were diluted in a solution of 0.1 M phosphate buffered saline (PBS; pH 7.2), 2.5% bovine serum albumin, 0,25% sodium azide and 2% triton X-100. Antisera specificity was determined in control experiments in which the primary antiserum was (1) omitted or (2) preabsorbed with an excess of anti-orexin-A+control peptide orexin-A (1:100) and anti-orexin-B+ control peptide orexin-B peptide (1:500). Fifteen rats were deeply anaesthetised with sodium pentobarbitone (150 mg/kg, i.p; Rh™ne M?rieux Harlow, UK) and perfused transcardially via the left ventricle with Krebs solution followed by 4% paraformaldehyde in 0.1M PBS. All subsequent steps were performed at room temperature unless indicated otherwise. Brain and spinal cord were removed, post-fixed with 4% paraformaldehyde in PBS (4-6 h), cryoprotected with 30% sucrose in PBS (16 h). Brain block was cut serially into 40µm thick coronal sections on a freeze knife microtome. Spinal cord sections were cut into 10µm in a sample order on a cryostat. Immunofluorescence protocol was used to visualize orexin immunoreactivity. Free-floating sections were washed five times with PBS (15 min per wash), incubated with 10% donkey serum in PBS (1 h) and washed once with PBS prior to incubation with anti-orexin-A (1:100) or anti-orexin-B (1:500) antiserum overnight at 4 0C. Sections were washed five times with PBS before incubation (1 h) with either biotinylated rabbit anti-species IgG as appropriate (1:500) then fluorescein (DTAF)conjugated streptavidin (1:400) (both from Jackson, U.S.A.) or anti-rabbit CY3 (1:150) (Jackson) and finally mounted on chrome alum gelatin-coated microscope slides and coverslipped with Vectashield (Vector, U.K.). Sections were examined by conventional fluorescence microscopy (596 nm excitation, 615 nm emission). A low magnification of coronal sections through the hypothalamus labeled with orexin-B antisera. Figure 1. Results Antisera specificity: To check the specificity of the orexin antisera, anti-orexin-A antiserum was preabsorbed with control peptide orexin-A and anti-orexin-B antiserum with control peptide orexin-B and omission of primary antibody. Immunoreactivity for orexin-A or orexin-B was not observed in the antiserum omission control experiment. Orexin-A and orexin-B immunoreactivities were abolished entirely by preabsorbtion with orexin-A or -B control peptide. Orexin-Bimmunoreactivity: Orexin-B immunoreactivity detected in A higher magnification showing the distribution of orexin-B-immunoreactive neurones and their processing in was the lateral hypothalamic area and perifornical nucleus. Figure 2. the cytoplasm of neurones that were restricted to the hypothalamus. Orexin-B immunoreactive neurones are concentrated bilaterally and symmetrically in the LHA and PeF, and more sparsely distributed medially in the dorsal aspects of the anterior hypothalamic area and the dorsomedial hypothalamic nucleus (DMH) (Fig. 1, 2). Morphologically, the majority of orexin-B positive neurones, which were similar in size and shape, appeared typically spherical and had several primary dendrites with few secondary branching (Fig. 2). Axons immunoreactive for orexin-B, were observed in the brain and spinal cord, were both varicose and non-varicose in appearance. In the hypothalamus, an extensive network of orexin-B immunoreactive fibres was observed in the LHA and PeF, in close apposition to the orexin-B containing neurones (Fig. 1, 2). Fibres immunoreactive for orexin-B were also A low magnification of coronal sections through the hypothalamus labeled with orexin-A antisera. Figure 3. A higher magnification showing orexin-Aimmunoreactive neurones and their processing are distributed in the lateral hypothalamic area and perifornical nucleus. Figure 4. abundant throughout much of the rest of the hypothalamus with moderately dense projections to the paraventricular nucleus, supraoptic nucleus, DMH, parastrial nucleus and preoptic areas. With the noticeable exception of arcuate nucleus, median eminence and other subregions of the hypothalamus where a fairly dense network of immunoreactive fibres is noted. Orexin-Bimmunoreactive fibres were abundant in the septal nuclei. In the thalamus, the midline nuclei exhibited a distinctive distribution of orexin-B-immunoreactive fibres. Orexin-B fibres were spread throughout the spinal cord at all levels investigated (cervical, thoracic, lumbar and sacral) with obvious localisation of fibres in the superficial dorsal horn. Orexin-Aimmunoreactivity: The distribution of orexin-A immunoreactive neurones in the LHA and PeF was similar to that of orexin-B (Fig. 3, 4). Fibres immunoreactive for orexin-A were also similar to that of orexinB in the brain (Fig 4) and spinal cord (Fig.5). Discussion With the use of antibodies specific against orexin-A or orexin-B peptides, the present immunocytochemical Photomicrograph showing the pattern of fluorescein-labelled orexin-A immuno-reactive fibres in lamina I-II of the spinal cord. Figure 5. investigation determined the distribution of orexincontaining neurones and processes in the rat brain and spinal cord. Populations of orexin-A- and orexin-Bimmunoreactive neurones were found in the hypothalamus concentrated in LHA/PeF region; orexin neurones were not found elsewhere in the rat brain and spinal cord. These findings are in agreement with recent studies on the localisation in the rat brain of neurones containing the orexin peptides (1, 3-7), or prepro-orexin-mRNA (1,5). With the exception of the testis, prepro-orexin mRNA has not been described elsewhere in the central or peripheral nervous systems (1). Both varicose and nonvaricose orexin-B fibres were distributed extensively throughout the hypothalamus but also projected to numerous other sites (e.g. the septal nuclei, the midline thalamic nuclei). A similar pattern of fibre distribution has been described recently in studies utilised antisera raised against the orexin-A, orexin-B, hypocretin-2 or preprohypocretin peptide sequences (5-7). Furthermore, we also report here the novel observations that orexin-A and orexin-B fibres are located at all levels of the spinal cord. Physiological studies with whole-cell voltage-clamp recording and digital imaging, have indicated that hypocretin receptors were located both on the cell body and on axon terminals (8). Finding that some spinal neurones respond to hypocretin application suggests that receptors may be expressed by some subpopulations of neurones. The hypothalamic area has been implicated in the regulation of feeding behaviour and energy homeostasis ever since the classic experiments showing that animals with lateral hypothalamic lesions had decreased food intake and lower set point for body weight (9-13). Since it has been demonstrated that orexin stimulates feeding (1,14) and orexin mRNA is increased with fasting (1), we shall focus on the possible participation of orexin in feeding. The hypothalamic area has been implicated in the regulation of feeding behaviour and energy homeostasis ever since the classic experiments showing that animals with lateral hypothalamic lesions had decreased food intake and lower set point for body weight (9-13). Since it has been demonstrated that orexin stimulates feeding (1,14) and orexin mRNA is increased with fasting (1), we shall focus on the possible participation of orexin in feeding. Our observations extend knowledge of these novel peptides in a number of ways. Firstly, orexin-A and orexin-B processes are located at all levels of the spinal cord with a pattern of expression similar to that found for hypocretin-2 (14). Secondly, when compared to the distribution of orexin-B immunoreactive fibres, those processes detected using orexin-A antiserum were similar pattern in many regions of the brain and the spinal cord. Despite the increasing understanding of the roles of the mediobasal hypothalamus in regulating feeding behaviour and energy homeostasis, the role of the LHA is still debated. The most important feature of the orexin system is that its neuronal cell bodies are localised only around the LHA and DMH and has a expansive projection. Our findings suggest that orexin neurones might receive information from the medial hypothalamus and other peripheral tissues, and send integrated information to outside of the hypothalamus, including the cerebral cortex, brainstem and limbic system. This distribution pattern is similar to that of melanin-concentrating hormone (MCH) neurones (15,16), but double staining studies has showed that these neurones are distinct from each other (17). This finding suggests that the orexin and MCH systems have a important roles in feeding behavior. The present study has demonstrated that the distribution of orexin cell bodies and fibres in the LHA and PeF region of the hypothalamus would allow them to participate in the control of feeding and energy balance. Our finding that orexin neurones are confined to the LHA region could support this contention since ~25% of LHA cells are glucose sensitive (stimulated by hypoglycaemia) and affected by high insulin levels (18,19). Thus, orexins may influence food intake by transmitting information that influences ingestive behavior from the hypothalamus to these regions. Further experimental investigation is needed to determine whether these neurones are immunoreactive for orexin. The morphological distribution of orexin-immunoreactive neurones and processes indicates that the orexins may participate in a host of other functions. For example, the LHA and perifornical area are reported to relate to cardiovascular responses to emotional situations or defensive behavior, and furthermore, orexin-A fibres were observed in nuclei that are involved in cardiovascular control such as the area postrema, the parabrachial nuclei, the locus coerulus, the nucleus of the solitary tract, the habenula and the periaqueductal gray (5,11,20). Finally, the LHA/ PeF area is also involved in the control of other processes such as fluid and temperature homeostasis, salivation, olfaction, pancreatic function and the regulation of autonomic function (11,12), and furthermore, these potentially diverse actions of orexin are supported by the extensive distribution of orexin fibres in the brain and spinal cord. In conclusion, the hypothalamus has a major role in regulating various behaviors that contribute to homeostasis such as arousal, feeding, and thermoregulation by integrating external and internal stimuli (21). Orexins, which have been discovered and characterised a family of neuropeptides, stimulate food consumption by acting on the lateral hypothalamus (1). Nahon (16) has discovered one other neuropeptide in the feeding centres of hypothalamus, MCH, that mediates feeding behavior. The localization of orexin cells bodies and processes to the LHA/PeF area suggests that the orexins may participate in the control of feeding and energy homeostasis. Findings of orexin fibres throughout much of the hypothalamus and at other sites in the brain, which have been implicated in feeding, are consistent with the appetite-stimulating properties of these peptides. As a result, the spread of the orexin fibres in the brain and spinal cord raises the possibility that the orexins may also be implicated in other vegetative and neuroendocrine regulations or related to general arousal states. References 1. Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., Williams, S.C., Richardson, J. A., Kozlowski, G. P., Wilson, S., Arch, J. R. S., Buckingham, R. E., Haynes, A. C., Carr, S. A., Annan, R. S., McNulty, D. E., Liu, W.-S., Terrett, J. A., Elshourbagy, N. A., Bergsma, D. J. and Yanagisawa, M.: Orexins and orexin receptors: A family of hypothalamic neuropeptides and G-protein-coupled receptor that regulate feeding behavior. Cell. 92: 573585, 1998. 2. Trivedi, P., Pu, H., McNeil, D. J., Van der Ploeg, L. H. T. and Guan, X.-M.: Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 438: 71-75, 1998. 3. Chen, C.-T., Dun, S. L., Kwok, E. H., Dun, N. J. and Chang, J.-K.: Orexin A-immunorectivity in the rat brain. Neurosci. Lett. 260: 161-164, 1999. 4. Nambu, T., Sakurai, T., Mizukami, K., Hosoya, Y., Yanagisawa, M. and Goto, K.: Distribution of orexin neurons in the adult rat brain. Brain Res. 827: 243-260, 1999. 5. DeLecea, L., Kilduff, T. S., Peyron, C., Gao, X.-B., Foye, P. E., Danielson, P. E., Fukuhara, C., Battenberg, E. L. F., Gautvick, V. T., Bartlett, F. S., Frankel, W. N., ven den Pol, A. N., Bloom, F. E., Gautvik, K. M. and Sutcliffe, J. G.: The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA 95: 322-327, 1998. 6. Peyron, C., Tighe, D. K., van den Pol, A. N., DeLecea, L., Heller, H. C., Sutcliffe, J. G. and Kilduff, T. S.: Neurones containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18: 9996-10015, 1998. 7. Date, Y., Ueta, Y., Yamashita, H., Yamaguchi, H., Matsukura, S., Kangawa, K., Sakurai, T., Yanagisawa, M. and Nakazato, M.: Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. USA 96: 748-753, 1999. 8. van den Pol, A. N., Gao, X. B., Obrietan, K., Kilduff, T. S. and Belousov, A.B.: Prepostsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin / orexin. J. Neurosci. 18(19): 7962-7971, 1998. 9. Bellinger, L. L.: Ingestive behavior of rats with ibotenic lesions of the dorsomedial hypothalamus. Am. J. Physiol. 252: R938-946, 1987. 10. Oomura, Y.: Input-output organisation in the hypothalamus relating to food intake behavior, in: Morgane, P. J. and Panksepp, J. (Eds.): Handbook of the hypothalamus, Volume 2: Physiology of the hypothalamus, Marcel Dekker, New York, 1980, pp. 557-620. 11. Bernardis, L. L. and Bellinger, L. L.: The lateral hypothalamic area revisited: neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci. Behav. Rev. 17: 141193, 1993. 12. Bernardis, L. L. and Bellinger, L. L.: The lateral hypothalamic area revisited: ingestive behavior. Neurosci. Behav. Rev. 20: 189-287, 1996. 13. Bernardis, L. L. and Bellinger, L. L.: The dorsomedial hypothalamic nucleus revisited: 1998 update, Proc. Soc. Exp. Biol. Med. 218: 284-306, 1998. 14. Lubkin, M. and Stricker-Kongrad, A.: Independent feeding and metabolic actions of orexins in mice. Biochem. Biophys. Res. Comm. 253: 241-245, 1998. 15. van den Pol, A.N.: Hypothalamic hypocretin (orexin): Robust innervation of the spinal cord. J. Neurosci. 19(8): 3171-3182, 1999. 16. Bittencourt, J.C., Presse, F., Arias, C., Peto, C., Vaughan, J., Nahon, J.-L., Vale, W. and Sawchenko, P.E.: The melanin-concentrating hormone system of the rat brain: an immunoand hybridisation histochemical characterisation. J. Comp. Neurol. 319: 218-245, 1992. 17. Nahon, J. L.: The melanin-concentrating gene: from peptide to the gene. Crit. Rev. Neurobiol. 8: 221-262, 1994. 18. Elias, C.F., Saper, C. B., Maratos-Flier, E., Tritos, N. A., Lee, C., Kelly, J., Tatro, J. B., Hoffman, G. E., Ollmann, M. M., Barsh, G. S., Sakurai, T., Yanagisawa, M. and Elmquist, J. K.: Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J. Comp. Neurol. 402: 442-459, 1998. 19. Oomura, Y., Ooyoma, H., Sugimoro, M., Nakamura, T. and Yamada, Y.: Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature, 247: 284-286, 1974. 20. Oomura, Y., and Yoshimatsu, H.: Neural network of the glucose monitoring system. J. Autonom. Nerv. Syst. 10: 359-372, 1984. 21. Smith, O.A., DeVito, J. L. and Astley, C. A.: Neurons controlling cardiovascular responses to emotion are located in the lateral hypothalamus—perifornical region. Am. J. Physiol. 259: R943-R954, 1990. 22. Simerly, R. B.: Anatomical substrates of hypothalamic integration. In: Paxinos, G, (ed.): The rat nervous system, 2nd Edition, Academic. 1995, pp. 353-376.