A graduate student was studying three different proteins X, Y and Z
advertisement

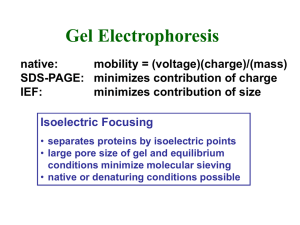
451A Assignment - 2 A graduate student was studying three different proteins X, Y and Z. He accidently mixed all three of them. Unfortunately he did not have any more stocks of these proteins in the lab. He/she thought of telling his mistake to his instructor but was afraid to disclose it. He/she thought about the nature of all three proteins and designed a strategy to separate them by simple biochemical techniques. Protein X is 167KDa, Y is 15KDa and Z is 13KDa. The student also knows that protein Y is a basic protein and protein Z has three FLAG epitope tag at its C-terminus. FLAG epitope consists of 8 amino acids and is used commonly to tag proteins for immunopurification. 1. Based on the above information, what method(s) should the student follow to separate X, Y and Z? Include in your answers different kinds and properties of resins the student should use. 2. Once purified, how would you check the quality and quantity of the purified proteins, and what should be the approach? 3. He also ran purified proteins on a non-denaturing gel. The result is shown below (left panel). Explain the gel and result. He than heated all three proteins. The result he observed is as shown on the gel (right panel). What do you think has happened to protein Z. Explain two different results obtained before and after heating protein Z. X Y X Z Y Z Heat proteins at 90C for 3mins before loading 4. He is interested in separating different forms of protein Z. What an analytical approach would you suggest to separated them and determine their molecular weight. 5. Explain why the molecular mass of fibrinogen is significantly overestimated when measured using a calibrated gel filtration column (see figure 6-10 in Voet & Voet), but can be determined with reasonable accuracy from its electrophoretic mobility on an SDSpolyacrylamide gel (see Table 6-5).