CHE499 TRANSPORT MODELING Spring 2007
advertisement
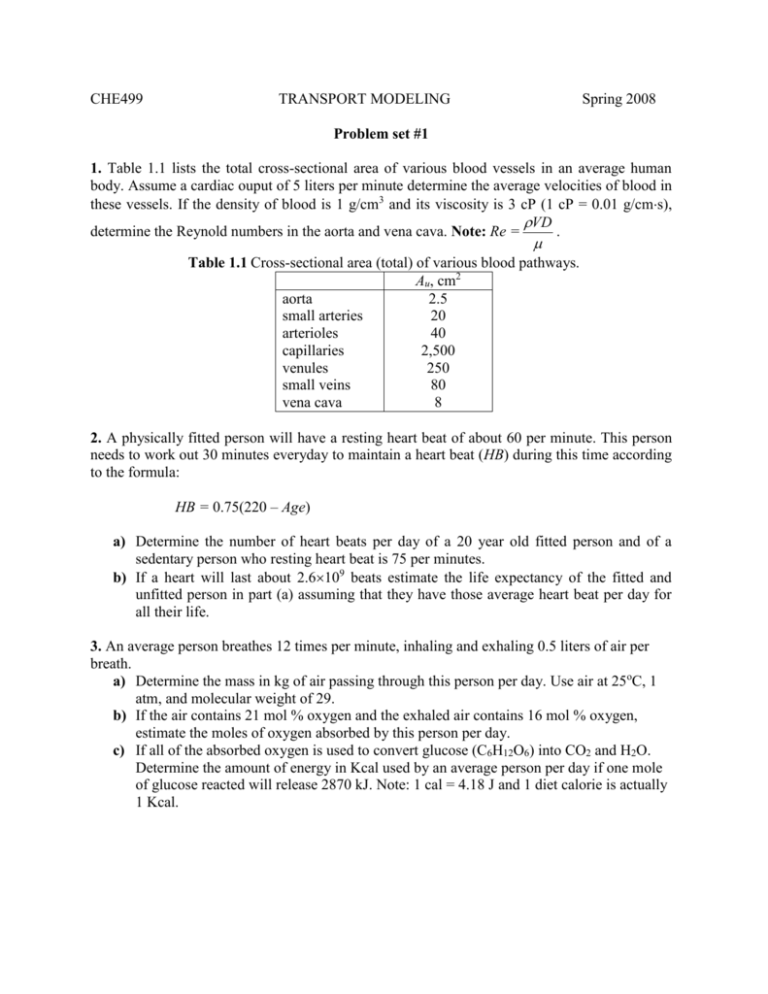
CHE499 TRANSPORT MODELING Spring 2008 Problem set #1 1. Table 1.1 lists the total cross-sectional area of various blood vessels in an average human body. Assume a cardiac ouput of 5 liters per minute determine the average velocities of blood in these vessels. If the density of blood is 1 g/cm3 and its viscosity is 3 cP (1 cP = 0.01 g/cms), VD determine the Reynold numbers in the aorta and vena cava. Note: Re = . Table 1.1 Cross-sectional area (total) of various blood pathways. Au, cm2 aorta 2.5 small arteries 20 arterioles 40 capillaries 2,500 venules 250 small veins 80 vena cava 8 2. A physically fitted person will have a resting heart beat of about 60 per minute. This person needs to work out 30 minutes everyday to maintain a heart beat (HB) during this time according to the formula: HB = 0.75(220 – Age) a) Determine the number of heart beats per day of a 20 year old fitted person and of a sedentary person who resting heart beat is 75 per minutes. b) If a heart will last about 2.6109 beats estimate the life expectancy of the fitted and unfitted person in part (a) assuming that they have those average heart beat per day for all their life. 3. An average person breathes 12 times per minute, inhaling and exhaling 0.5 liters of air per breath. a) Determine the mass in kg of air passing through this person per day. Use air at 25oC, 1 atm, and molecular weight of 29. b) If the air contains 21 mol % oxygen and the exhaled air contains 16 mol % oxygen, estimate the moles of oxygen absorbed by this person per day. c) If all of the absorbed oxygen is used to convert glucose (C6H12O6) into CO2 and H2O. Determine the amount of energy in Kcal used by an average person per day if one mole of glucose reacted will release 2870 kJ. Note: 1 cal = 4.18 J and 1 diet calorie is actually 1 Kcal. 4. 1Assume that air is an ideal gas, calculating the change in entropy and enthalpy when 100 moles of air at 25oC and 1 atm is heated and compressed to 300oC and 10 atm. Use the following equation for Cp Cp R = A + BT + CT2 where A = 3.355, B = 0.57510-3, and C = 0.016105, with T in K. R = 8.314 J/molK 5. 2You have a summer job with a company that is interested in building a plant to manufacture the painkiller ibuprofen [2-(p-isobutylphenyl) propionic acid, C13H18O2] using a new reaction scheme. Your assignment is to collect some data on one of the reactions, as a first step in designing a full-scale reactor. In one experiment, you mix 134 g isobutylbenzene (IBB, C10H14) with 134 g acetic anhydride (AAn, C4H6O3) in a laboratory-scale batch reactor, adjust the temperature, and wait 1 hour. At the end of the hour you stop the reaction, collect all the material in the pot, and send it for chemical analysis. The report come back that the pot contains IBB, acetic anhydride, isobutylacetophenone (IBA, C12H16O) and acetic acid (Aac, CH3COOH). Unfortunately, someone spilled coffee on the report and all you can read is the amount of isobutylbenxene: 1.6 g. Your boss is upset-he needs the data right away. Can you determine the amounts of IBB, AAn, and IBA from the available information for your boss? 6. 1Calculate the work required to compress adiabatically 60 moles of carbon dioxide initially at 25oC and 1 atm to 350oC and 10 atm. Assume that Cp = 37.1 J/molK. If the process is carried out reversibly, how much work is required? Assume that carbon dioxide is an ideal gas. 1 2 Fournier, R. L., “Basic Transport Phenomena in Biomedical Engineering”, Taylor & Francis, 2007, p. 85 Murphy, R. M., “Introduction to Chemical Processes Principles, Analysis, Synthesis”, McGraw Hill, 2007, p. 96











