standing order - Van Buren/Cass District Health Department
advertisement

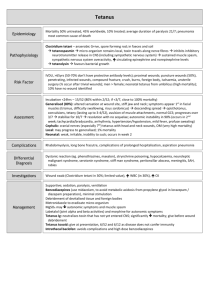
VAN BUREN/CASS DISTRICT HEALTH DEPARTMENT TETANUS - DIPHTHERIA (Td) VACCINATION STANDING ORDER INDICATIONS To prevent tetanus, which is an acute, often fatal, disease caused by an exotoxin produced by Clostridium tetani. a. Td may be administered for individuals 7 years of age and older. DPT, or DT if pertussis vaccine is contraindicated, may be given for children less than age 7 years old. b. Td is given for wound management if client has less than 3 doses of tetanus toxoid which includes clean, minor, arid all other wounds. c. SUMMARY GUIDE TO TETANUS PROPHYLAXIS IN ROUTINE WOUND MANAGEMENT, 1991 History or Adsorbed Tetanus Clean, Minor Wounds All Other Wounds Toxoid (doses) Td *TIG Td *TIG Unknown or < 3 Yes No Yes Yes 3 or more doses No No§ No No * ** § Tetanus Immune Globulin Such as, but not limited to, wounds contaminated with dirt, feces, soil, and saliva, puncture wounds; avulsion; and wounds resulting from missiles, crushing, bums, and frostbite. Yes, If > 10 years since last dose. Yes, if > 5 years since last dose. (More frequent boosters are not needed and can accentuate side effects.) Note: Td contains one-third as much diphtheria toxoid as DT. d. Recommended at age 11 - 12 years for a routine booster then every 10 years DOSAGE AND ADMINISTRATION 3 doses of Td is a primary series. 2 doses of 0.5cc of Td IM at least 4 weeks apart; followed by 0.5cc Td vaccine IM 6-12 months after 2nd dose. Td Vaccination Standing Order, Page 1 of 2 Routine Diphtheria and Tetanus Vaccination Schedule Summary for Persons > 7 Years of Age Dose Primary 1 Primary 2 Primary 3 Booster Age/Interval First dose 0.5 cc IM 4-8 weeks after first dose 0.5 cc IM 6-12 months after second dose 0.5 cc IM Every 10 years after last dose Product Td Td Td Td CONTRAINDICATIONS a. Pregnant women in their first trimester b. History of severe local reaction (Arthus-type) or fever > 103oF following previous dose c. History of neurologic or severe allergic reaction (acute respiratory distress or collapse) following a previous dose of tetanus toxoid. d. Hypersensitivity to thimerosal (mercury derivative). REFERENCES 1. Connaught Laboratories Inc. Product Information 6-1996. U.S.A. 2. Epidemiology and Prevention of Vaccine Preventable Diseases, Dr. William Atkins, 1998. 2nd printing. 3 CDC US Department of Health and Human Services P.H.S. Atlanta, GA. DISTRIBUTION Immunization Manual John R. Spriegel, M.D., M.P.H. Medical Director Rev: 9/17/02 StandingOrders/TdVaccination.doc Td Vaccination Standing Order, Page 2 of 2 Date Swiftwater, PA. 18370