Tetanus Prophylaxis in Children
advertisement

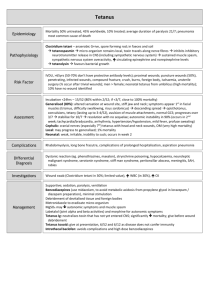
Paediatric Clinical Guideline Emergency 1.5 Tetanus Prophylaxis Short Title: Tetanus Prophylaxis Full Title: Date of production/Last revision: Guideline for the management of a tetanus prophylaxis in children and young people January 2007 Explicit definition of patient group to which it applies: This guideline applies to all children and young people under the age of 19 years. Name of contact author Dr Leonie Wong Paediatric Specialist Registrar Dr Stephanie Smith Consultant Emergency Paediatrician Ext 64042 Revision Date January 2010 This guideline has been registered with the Trust. However, clinical guidelines are 'guidelines' only. The interpretation and application of clinical guidelines will remain the responsibility of the individual clinician. If in doubt contact a senior colleague or expert. Caution is advised when using guidelines after the review date. Tetanus Prophylaxis in Children Introduction Tetanus is caused by the action of tetanus toxin following infection by Clostridium tetani. C. tetani is transmitted by soil or faecal contamination of wounds and has an incubation period of between 4 and 21 days (commonly 10). Tetanus is characterised by generalised skeletal muscle rigidity and spasms usually affecting the neck and jaw. There were 198 cases of tetanus reported in England and Wales between 1984 and 2004. The elderly are at highest risk. There were no reported cases in children under 5 years. Tetanus immunisation was introduced nationally as part of the primary immunisation programme in 1961. Most children receive their primary immunisations at 2, 3 and 4 months, with boosters at 4 years and 14-15 years. These are administered via their General Practitioner, community clinic or school. Leonie Wong Page 1 of 5 January 2007 Paediatric Clinical Guideline Emergency 1.5 Tetanus Prophylaxis Management of Tetanus Prone Wounds 1. Check the child’s tetanus immunisation status The normal schedule is 3 doses of DTaP/IPV/HiB given at 2, 3 and 4 months as part of primary immunisations Reinforcing (booster) doses at 3.5-5yrs of age 13-15yrs of age It is essential to check the immune and vaccination status of any patient presenting to the hospital (via A&E or direct transfer) with ANY wound or burn, however trivial at any interval. 2. Consider is this a tetanus prone wound? The following are considered tetanus-prone wounds: Wounds / burns requiring surgical intervention that is delayed for > 6hours Significant degree of devitalised tissue Wounds where there has been contact with soil or manure Puncture-type injury Compound fractures Wounds containing foreign bodies Wounds / burns in patients with systemic sepsis 3. Wound toilet, dressing and antibiotics as required Thorough surgical toilet of the wound is essential irrespective of the immunisation history of the patient and appropriate antibiotics should be prescribed. 4. Use the table to determine whether immunisation or human tetanus immunoglobulin is required? Immunisation Status Fully immunised1 Primary immunisation complete, boosters incomplete but up to date Primary immunisation incomplete or boosters not up to date Not immunised or immunisation status not known / uncertain3 Clean Wound Vaccine Not required Not required Reinforcing dose and further doses to complete recommended schedule Immediate dose of vaccine followed by completion of full 5dose course Tetanus-prone Wound Vaccine HTIG Not required Only if high risk2 Not required Only if high risk2 Reinforcing dose and further doses to complete recommended schedule Immediate dose of vaccine followed by completion of full 5dose course Yes (given at different site to vaccine) Yes (given at different site to vaccine) Notes 1. Fully immunised: Has received total of 5 doses of vaccine at appropriate intervals 2. High Risk: Heavy contamination with material likely to contain tetanus spores and/or extensive devitalised tissue 3. Immunosuppressed patients presenting with a tetanus prone wound should always be managed as if they were incompletely immunised. Please refer to the RCPCH guidance on Immunisation of the Immunocompromised Child Leonie Wong Page 2 of 5 January 2007 Paediatric Clinical Guideline Emergency 1.5 Tetanus Prophylaxis 5. Ensure patient receives tetanus immunisation where indicated Patients requiring a tetanus booster should have it administered in the department. Patients who have not received primary immunisation: Contact GP and arrange appointment for primary immunisation If appointment not available within 36 hours, give adsorbed vaccine as required in the department and refer to GP to complete the primary course. If parents refuse immunisation, inform GP and record your actions in the notes. Human Tetanus Immunoglobulin Indications Treatment of clinically suspected cases of tetanus (a notifiable disease) Prevention of tetanus in high risk tetanus prone wounds (see table and note above) Dose Available in 1ml ampoules containing 250IU Prevention Dose Treatment Dose 250IU by IM injection or 500IU by IM injection if >24 hours since injury / risk of heavy contamination / burns 5000 – 10000IU by IV infusion Or 150IU/kg by IM injection (given in multiple sites) if IV preparation unavailable Contraindications Confirmed anaphylactic reaction to tetanus containing vaccine Confirmed anaphylactic reaction to neomycin, streptomycin or polymyxin B Adverse Reactions Local Pain, erythema, induration (Arthus type reaction) General Pyrexia, hypotonic-hyporesponsive episode, persistent crying Confirmed anaphylaxis is very rare (0.65 – 3 events per million doses) All suspected adverse reactions should be reported to the Committee on Human Medicines using the Yellow Card scheme. Leonie Wong Page 3 of 5 January 2007 Paediatric Clinical Guideline Emergency 1.5 Tetanus Prophylaxis Tetanus Vaccine Tetanus vaccine is only available in combination with other vaccines. When a reinforcing dose is required the choice of the vaccine will depend on the age of the child and their immunisation status. Children <10yrs Children >10 yrs of age Leonie Wong Incomplete/delayed primary vaccination The primary course is three doses of a tetanus-containing vaccine (DTaP/IPV/Hib) with an interval of one month between doses This can be given at any stage from 2 months-10yrs If the primary course is interrupted it should be resumed but not repeated Three doses of 0.5mls Td/IPV with one month between doses Page 4 of 5 Incomplete/delayed reinforcing doses Give a single dose of 0.5mls DTaP/IPV or dTaP/IPV im This will re-establish the child on the routine schedule A single dose of 0.5mls Td/IPV This will re-establish the child on the routine schedule January 2007 Paediatric Clinical Guideline Emergency 1.5 Tetanus Prophylaxis References Immunisation against Infectious Disease, 3rd edition, Department of Health, 2006. – The Green Book Immunisation of the Immunocompromised Child. Best Practice Statement. RCPCH. 2002 Title Tetanus Prophylaxis Guideline Number 1.5 Version Final Distribution All wards QMC and CHN Author Dr Leonie Wong Paediatric Specialist Registrar Dr Stephanie Smith Consultant Emergency Paediatrician Document Derivation Immunisation against Infectious Disease, 3rd edition, Department of Health, 2006. – The Green Book Immunisation of the Immunocompromised Child. Best Practice Statement. RCPCH. 2002 First Issued November 1997 Latest Version Date January 2007 Review Date January 2010 Ratified By Paediatric Clinical Guidelines Meeting Date January 2007 Audit Amendments Leonie Wong Induction Programme Page 5 of 5 January 2007