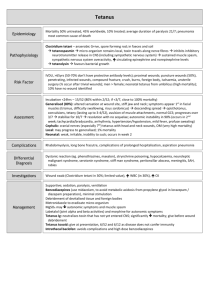
TETANUS IMMUNIZATION Ov erv ie w T etanus is a potentially fatal noncommunicable disease caused by the toxin (tetanospasmin). It is produced by the spore-forming bacteria Clostridium tetani, an anaerobic Gram-positive bacillus. The spores are hardy, resistant to heat and antiseptics, and found ubiquitously in the soil and feces of humans and animals. Successful treatment depends on proper care and treatment of wounds and traumatic injuries and prevention through appropriate tetanus immunization. Worldwide, tetanus still accounts for 1 million hospital admissions. Most of these cases are in Africa and Southeast Asia, but they are decreasing with immunization initiatives directed to these areas. In 2012, tetanus caused 213,000 deaths worldwide. Most of these deaths occurred in developing countries, and one-half were in neonates. Mortality in these areas remains high (30% to 70%). In industrialized countries, mortality from tetanus is lower. The CDC reports case fatality of 13.2% in the United States. Tetanus is almost entirely preventable with adequate immunization. The disease has been central to the World Health Organization (WHO) Expanded Programme on Immunization since 1974. The incidence of tetanus decreases when immunization programs are in place. Unfortunately, under-immunized populations exist even in high-income countries. During the surveillance period of 2001–2008 in the United States, 233 cases associated with 26 deaths were reported. Individuals over the age of 50 represented one-half of those cases, and individuals over 65 represented 30% of the cases. Death was five times more likely in people older than 65. Older women are particularly at risk, because most of those over age 55 do not have protective levels of tetanus antibody. Diabetics and injection drug users are other high-risk groups. Tetanus can occur in nonacute wounds, and 1 of 6 cases surveyed was associated with non-acute wounds. Inadequate tetanus toxoid vaccination and inadequate wound prophylaxis are the most important factors associated with the development of tetanus. Tetanus surveillance data have demonstrated two interesting findings: Fewer than 4% of those with acute wounds who sought treatment received appropriate prophylaxis. Only 36.5% sought immediate medical care for their wounds. All medical professionals must be cognizant of these factors when providing care to injured patients. Tetanus immunization depends on the patient’s previous immunization status and the tetanus-prone nature of the wound. The following guidelines are adapted from the literature, and information is available from the Centers for Disease Control and Prevention (CDC). Because this information is continuously reviewed and updated as new data become available, the American College of Surgeons Committee on Trauma recommends contacting the CDC for the most current information and detailed guidelines related to tetanus prophylaxis and immunization for injured patients. National guidelines may vary. Pathoph ysiolo g y Clostridium tetani spores are found in the soil and in the feces of animals and humans. The spores access the body through breaks in the skin and grow under low oxygen conditions. Wounds that tend to propagate spore development are typically puncture wounds and wounds with significant tissue destruction. Tetanospasmin causes tetanus by blocking inhibitory pathways (gamma-aminobutyric acid), producing sustained excitatory nervous impulses that give rise to the typical clinical symptoms. Once the spores gain access to the body through an open wound, they undergo an incubation period of from 1 to 2 days and as long as 7 to 21 days. The diagnosis is usually clinical, and the treatment is supportive. Prevention is the mainstay of treatment. Types of wounds likely to encourage the growth of tetanus organisms include •• Open fractures •• Deep penetrating wounds (> 1 cm) •• Stellate or avulsion configuration •• Wounds containing devascularized tissue •• Wounds resulting from a missile (gunshot wound) •• Wounds from burns or frostbite 21 ­22 TETANUS IMMUNIZATION •• Wounds containing foreign bodies (especially wood splinters) •• Wounds complicated by pyogenic infections •• Wounds with extensive tissue damage (e.g., contusions or burns) •• Any wound obviously contaminated with soil, dust, or horse manure (especially if topical disinfection is delayed more than 4 hours) •• Reimplantation of an avulsed tooth (because the tooth receives minimal washing and cleaning to increase the likelihood of successful reimplantation) •• Wounds or burns requiring surgical intervention that is delayed more than 6 hours •• Wounds or burns associated with sepsis Wounds must be cleaned, disinfected, and treated surgically if appropriate. Clinical Signs and Course The excitatory impulses lead to sustained muscular contractions, which can be localized or generalized. Contractions may begin in the muscles surrounding the wounded area. Lockjaw (severe contraction of the masseter muscle) is characteristic of generalized tetanus. Pain, headache and muscle rigidity are seen in generalized tetanus (80% of cases). Respiratory failure caused by laryngeal obstruction and chest wall rigidity is the most common direct cause of death. Autonomic dysfunction can be seen as well with accompanying fever, diaphoresis, hypertension, arrhythmias, and hypermetabolism. The spasms and autonomic instability persist for weeks, and the muscular rigidity is present for months. Tr e atment Pr inc iple s the risk for tetanus infection in soft-tissue wounds are detailed in n TABLE 1. However, clinicians should consider all wounds to be at risk for the development of tetanus. Prevention Active immunization is the mainstay of therapy for this disease. The following general principles for doctors who treat trauma patients concern surgical wound care and passive immunization. Studies demonstrate that relying on patients to recall their immunity status may be unreliable, resulting in both over- and under-administration of tetanus boosters. Over-administration of tetanus prophylaxis may diminish serologic response and increase cost of care, whereas under-treatment exposes patients to the risk of developing the disease and risking mortality and morbidity. Serologic testing is available to determine antibody levels. n BOX 1 lists potential adverse reactions from tetanus immunization. Passive Immunization Passive immunization with 250 units of human tetanus immune globulin (TIG), administered intramuscularly, must be considered for each patient. Double the dose if the wound is older than 12 hours, there is heavy contamination, or the patient weighs more than 90 kg. TIG provides longer protection than antitoxin of animal origin and causes few adverse reactions. Characteristics of the wound, the conditions under which it occurred, wound age, TIG treatment, and the patient’s previous active immunization status must all be considered. Due to concerns about herd immunity to both pertussis and diphtheria, and recent outbreaks of both, box 1 adverse reactions from tetanus immunization • Pain • Palpable lump Surgical Wound Care • Swelling Regardless of a patient’s active immunization status, he or she must immediately receive meticulous surgical care—including removal of all devitalized tissue and foreign bodies—for all wounds. If the adequacy of wound debridement is in question or a puncture injury is present, leave the wound open and do not suture. Such care is essential as part of the prophylaxis against tetanus. Traditional clinical features that influence • Type II hypersensitivity reaction with severe swelling • Erythema at the injection site occurring in up to 20% and erythema of the injected arm within 2 to 8 hours of the injection. (It usually resolves without sequelae.) • General symptoms of malaise fever headache are uncommon; dyspnea, urticaria, angioedema, and neurologic reactions are rare. • Anaphylaxis 0.6 to 3 per million doses TETANUS IMMUNIZATION Dr. Henry: Please add title to the table 23 table 1 title AGE (YEARS) 0 through 6 VACCINATION HISTORY Unknown or not up-to-date on DTaP DTaP DTaP series based on age 7 through 10 ALL OTHER WOUNDS CLEAN, MINOR WOUNDS TIG Up-to-date on DTaP series based on age No indication No indication Unknown or incomplete DTaP series Tdap and recommend catch-up Tdap and recommend vaccination catch-up vaccination TIG Completed DTaP series AND <5 years No indication No indication No indication Td, but Tdap preferred since last dose Completed DTaP series AND ≥ 5 years since last dose 11 and older if child is 10 years of age Unknown or <3 doses of tetanus Tdap and recommend catch-up Tdap and recommend toxoid containing vaccine vaccination catch-up vaccination TIG (*if pregnant, see footnote) 3 or more doses of tetanus toxoid No indication No indication No indication Tdap preferred (if not containing vaccine AND <5 years since last dose 3 or more doses of tetanus toxoid containing vaccine AND 5-10 years yet received) or Td since last dose 3 or more doses of tetanus toxoid Tdap preferred (if not yet Tdap preferred (if not containing vaccine AND >10 years since received) or Td yet received) or Td last dose Dr. Henry: Please add source information. *Pregnant Women: As part of standard wound management care to prevent tetanus, a vaccine containing tetanus toxoid might be recommended for wound management in a pregnant woman if 5 years or more have elapsed since the previous Td booster. If a Td booster is recommended for a pregnant woman, health care providers should administer Tdap. Tdap (tetanus, diphtheria, and pertussis) is preferred to Td (tetanus and diphtheria) for adults who have never received Tdap. Td is preferred to TT (tetanus toxoid) for adults who received Tdap previously or when Tdap is not available. If TT and TIG are both given, administer tetanus toxoid adsorbed rather than tetanus toxoid for booster use only (fluid vaccine). When tetanus toxoid and TIG are given concurrently, use separate syringes and separate sites. If the patient has ever received a series of three injections of toxoid, TIG is not indicated, unless the wound is judged to be tetanus-prone and is more than 24 hours old. Table 1 outlines age-based recommendations for vaccination considering vaccination history and wound type, and n FIGURE 1 provides a summary guide of tetanus prophylaxis in routine wound management. Bibliography Dr. Henry: is this the appropriate place to cite the table and figure? 1. Advisory Committee on Immunization Practices. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory ­24 TETANUS IMMUNIZATION Summary Guide to Tetanus Prophylaxis in Routine Wound Management ASSESS WOUND A clean, minor wound All other wounds (contaminated with dirt, feces, saliva, soil; puncture wounds; avulsions; wounds resulting from flying or crushing objects, animal bites, burns, frostbite) Has patient completed a primary tetanus diphtheria series?1,7 Has patient completed a primary tetanus diphtheria series?1,7 Yes No/Unknown 2,3,4 Administer vaccine today. Instruct patient to complete series per age-appropriate vaccine schedule. Was the most recent dose within the past 10 years? No Administer vaccine today.2,4 Patient should receive next dose per age-appropriate schedule. 1 2 3 Yes No/Unknown Yes No *Tdap vaccines: Adacel (Sanofi) is licensed for persons 11 through 64 years of age. Boostrix (GSK) is licensed for persons 10 years of age and older. 1 Brand names are used for the purpose of clarifying product characteristics and are not in any way an endorsement of either product. Yes Administer vaccine today.2,4 Patient should receive next dose per age-appropriate schedule. Vaccine not needed today. Patient should receive next dose at 10-year interval after last dose. A primary series consists of a minimum of 3 doses of tetanus- and diphtheriacontaining vaccine (DTaP/DTP/Tdap/DT/Td). Age-appropriate vaccine: DTaP for infants and children 6 weeks up to 7 years of age (or DT pediatric if pertussis vaccine is contraindicated); Tetanus-diphtheria (Td) toxoid for persons 7 through 9 years of age; and ≥65 years of age; Tdap for persons 10 through 64 years of age if using Adacel1 or 10 years of age and older if using Boostrix1, unless the person has received a prior dose of Tdap.* No vaccine or TIG is recommended for infants <6 weeks of age with clean, minor wounds. (And no vaccine is licensed for infants <6 weeks of age.) Was the most recent dose within the past 5 years?7 Administer vaccine and tetanus immune gobulin (TIG) now.2,4,5,6,7 4 5 6 7 Vaccine not needed today. Patient should receive next dose at 10-year interval after last dose. Tdap* is preferred for persons 10 through 64 years of age if using Adacel1 or 10 years of age and older if using Boostrix1 who have never received Tdap. Td is preferred to tetanus toxoid (TT) for persons 7 through 9 years of age, or ≥65 years of age if only Adacel1 is available, or those who have received a Tdap previously. If TT is administered, an adsorbed TT product is preferred to fluid TT. (All DTaP/DTP/Tdap/DT/Td products contain adsorbed tetanus toxoid.) Give TIG 250 U IM for all ages. It can and should be given simultaneously with the tetanus-containing vaccine. For infants <6 weeks of age, TIG (without vaccine) is recommended for “dirty” wounds (wounds other than clean, minor). Persons who are HIV positive should receive TIG regardless of tetanus immunization history. Immunization Program P.O. Box 64975 St. Paul, MN 55164-0975 651-201-5414, 1-877-676-5414 www.health.state.mn.us/immunize (9/12) IC# 141-0332 n FIGURE 1 Summary Guide to Tetanus Prophylaxis in Routine Wound Management. Reprinted from Minnesota Department of Health Immunization Program. Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR 2006;December 15;55(RR-17):1–37. 2. Bakole I, Danesi M, Oluwasdamilola O, et al. Characteristic and outcome of tetanus in adolescent and adult patients admitted to the Lagos University Teaching Hospital between 2000 and 2009. J Neurol Sci 2012;323: 201–204. 3. Centers for Disease Control (CDC). Tetanus surveillance—United States, 2001–2009. MMWR 2011;60:365–396. 4. CDC. Updated recommendations for use of tetanus toxoid reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR 2011;60:13–15. 5. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR 2012;61:468–470. 6. CDC. Updated recommendations for the use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR 2013;62:131–135. 7. Collins S, White J, Ramsay M, et al. The importance of tetanus risk assessment during wound management. ID Case Rep 2015;2:3–5. 8. Laurichesse H, Zimmermann U, Galtier F, et al. Immunogenicity and safety results from a randomized multicenter trial comparing a Tdap-IPV vaccine (REPEVAX®) and a tetanus monovalent vaccine in healthy adults: new considerations for the management of TETANUS IMMUNIZATION patients with tetanus-prone injuries. Human Vaccines & Immunotherapeutics 2012;8:12: 1875–1881. 9. McVicar, J. Should we test for tetanus immunity in all emergency department patients with wounds? Emerg Med J 2013;30: 177–179. 25 10. Rhee P, Nunley MK, Demetriades D, et al. Tetanus and trauma: a review and recommendation. J Trauma 2005;58:1082–1088. 11. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Tetanus. https://www.cdc.gov/vaccines/vpd/ tetanus/index.html