Chemistry Equilibrium Worksheet
advertisement

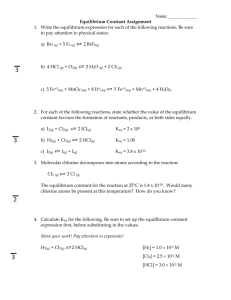
Chemistry Equilibrium Worksheet Block:_______ Name:__________KEY________________ 1. Consider the balanced, reversible reaction: CO (g) + 3H2 (g) → CH4 (g) + H2O (g). ∆H= -206.1 kJ a. Is this reaction EXOthermic or ENDOthermic? _ Exo_ b. Which side had higher entropy (disorder)? _Left___ Left side has more moles of gases. c. Which way (to L or R?) does the tendency towards minimizing potential energy drive this reaction? __Right____ d. Which way does the tendency to maximize entropy drive the reaction? __ Left___ e. Write the equilibrium constant (mass action) expression for this reaction. Keq = [CH4][H2O]_ [CO][H2]3 f. At equilibrium at a particular temperature, Keq for this reaction is 3.92. If we mix enough gases so that the mixture is 1 M [CO], 1 M [H 2], 1 M [CH4], and 1 M [H2O], at this temperature, will the system be at equilibrium? No How do you know? Reaction Quotient does not equal to the Keq Q = (1)(1)/(1)(1)3 Right Why? The system will tend to go to the equilibrium: increasing the reaction quotient to make it equal to the Keq. To do this, the reaction has to go to the right to produce more products (see the Keq expression). If the system is not at equilibrium with that mixture in (f), will it spontaneously react to the left or the right? g. If the system is at equilibrium at some particular set of conditions, which way will it react if some additional CO is added? Right h. If the system is at equilibrium at some particular set of conditions, which way will it react if some CH4 is removed? Right i. If the system is at equilibrium at some particular set of conditions, which way will it react if the system is squeezed into a smaller volume, raising the pressure, with the temperature kept constant? Right j. If the system is at equilibrium at some particular set of conditions, which way will it react if the temperature is raised? Left k. If the system is at equilibrium at some particular set of conditions, which way will it react if a material is added that serves as a catalyst for this reaction? _______ l. For each of the 5 previous questions (dealing with the use of LeChatelier’s Principle), tell what will be the change in Keq for each change; will K increase, decrease, or remain unchanged? Keq will: g. Unchanged h. Unchanged i. Unchanged j. Decrease k.Unchanged m. At a temperature at which Keq= 4.0, what must be the [CO] if; [H2]= 2.0M, [CH4] = 5.0M, and [H2O] = 3.0M? [CO]= 0.47 M Keq = (5.0)(3.0) = 4.0 x(2.0)3 x = 0.469 n. 4.0 moles of CO and 2.0 moles of H2 were mixed in a 1.0 L container, and kept at constant temperature until equilibrium was reached. At equilibrium, it was found that 3.4 moles of CO were still in the container. Solve for all of the “?” Substance CO H2 CH4 H2O Initially (mol/L) 4.0 2.0 ? ? 0 0 Change ? - 0.6 ? - 1.8 ? + 0.6 ? + 0.6 @ Equilibrium (mol/L) 3.4 ? ? ? 0.2 0.6 0.6 a. Determine the concentration of all the substances @ equilibrium: Keq = (0.6)(0.6) = 13.2 (3.4)(0.2)3 b. Solve for the Keq = Was this experiment done at higher, lower, or the same temperature as that in m., above? 10 Lower Explain Keq = 10 is greater than Keq = 4.0 It means more reactants are present, left side is favored. For the exothermic reaction it means lower temperature (when temperature is decreased, the exothermic reaction shifts left). o. In another experiment, 2.0 atm. of CO, 1.5 atm. of H 2, and 2.5 atm. of H2O were mixed and equilibrated at constant temperature in a container. The net reaction must have been to the Right. No CH4 is present, the reaction has to go right to produce it. At equilibrium there were 1.7 atm. of CO remaining. Therefore, at equilibrium; [CO] = 1.7 atm. [H2] = _0.6_ atm, [CH4] = _0.3_ atm, [H2O] = _2.8_ atm, and Kp = _2_. (This is Kp because it uses pressures—before we’ve found Kc’s, using concentrations.) Substance Initially (atm) Change @ Equilibrium (atm) Kp = (0.3)(2.8) = 2.3 CO 2.0 ? - 0.3 1.7 (1.7)(0.6)3 H2 1.5 ? - 0.9 ? 0.6 CH4 ? 0 ? + 0.3 ? 0.3 H2O ? 2.5 ? + 0.3 ? 2.8