EQUILIBRIUM
advertisement

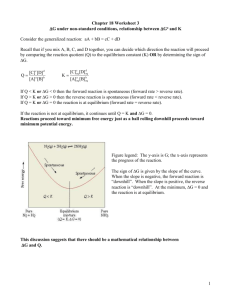
Teacher Notes - TOPIC 1 - Equilibrium Up to now in this course you may have had the impression that chemical reactions proceed in the following manner: First, reactants are mixed. Next, they react until all of the reactants are used up and only products are present. The reaction then stops. In fact, most reactions do not proceed like this. Often, equilibrium is established during a chemical reaction. o Most reactions DO NOT go to completion o Reactions that do not go to completion are REVERSIBLE o Reversible reactions exist in a state of EQUILIBRIUM What is equal about equilibrium? Show DEMO: Equilibrium (aquariums or two large clear containers) Equilibrium in chemical systems is defined as the point at which the rate of the forward reaction is equals to the rate of the reverse reaction: Rate forward rxn = Rate reverse rxn (NO change in amounts of R or P) In other words, products are forming as rapidly as they are being used up to make reactants again. At this point, the ratio of products to reactants is not necessarily equal to one! The ratio of products to reactants at this point is not changing; it is constant. This ratio is called the equilibrium constant (Keq). Again, the equilibrium constant is defined as the ratio of products to reactants. More specifically, it is the ratio of products, raised to their coefficients, to reactants, raised to their coefficients. Let’s look at the reaction below. o aA + bB cC + dD The equilibrium constant expression for this reaction would be: coefficient Keq = [C]c [D]d [A]a [B]b Keq = [C]c [A]a [B]b **If Keq >1, products are favored; If Keq<1, reactants are favored. For this reaction, aA + bB Ex. H2(g) + I2(g) 2 HI(g) at 25oC cC , Keq = 49.7 >1 (more products) Remember: liquids (l) and solids (s) are pure substances, therefore their concentrations are constant at a given T’. That’s b/c the concentration of a pure substances is its density in moles per liter. At any given T’, density does not change. No matter how much liquid or solid is present, its concentration remains constant. Therefore, concenrations of (l) and (s) are constant and can be combined with K - don’t include them in the equations. The value of Keq is given without units. Ex. FeO(s) + CO(g) Fe(s) + CO2(g) Keq = [ CO2] [ CO ] Examples 1. Write equilibrium expressions for each of the following reactions. A) H2(g) + F2(g) 2HF(g) B) 4NO(g) C) HCN(aq) + 3O2(g) H+ (aq) + 2N2O5 (g) CN- (aq) 2. Given this reaction, H2 + I2 2HI, and these equilibrium concentrations, [H2] = 1.14 x 10–3 M and [I2] = 1.14 x 10-3 M, and [HI] = 8.41 x 10-3 M, find the equilibrium constant. Keq = _______________________ 3. If the following reaction reaches equilibrium and the concentrations are as given below, find the concentration of the CH4. CO(g) + 3 H2(g) CH4(g) + H2O(g) Keq = 3.933 [CO] = 0.85 M [H2O] = 0.286 M [H2] = 1.333 M [CH4] = ?