Laboratory 4

LAB 4:
Analysis of Mouthwash Experiment and EMB Plating
Results & Determining Concentration of
Bacterial Cultures
I. Objectives
In today’s lab, students will learn how to read and interpret a Kirby-Bauer test. Students will also learn how to interpret the results from their EMB plating experiment and how to determine the number of cells per mL in a bacterial culture. By the end of today’s lab, students should:
Be able to read and interpret the results of a Kirby Bauer test
Know how to identify coliforms and E. coli colonies on EMB agar plates based on colony morphology
Understand why scientists perform serial dilutions
Know how to perform a serial dilution
Know how to calculate the concentration of cells in a bacterial culture using serial dilutions and plating
II. Safety considerations
Wear gloves when handling bacteria and performing serial dilutions
Do not touch the colonies on any of the plates
Leave the lids on the plates at all times
III. Introduction
A. ANTIBIOTICS
In your Kirby-Bauer test, you used an antibiotic called ciprofloxacin as a positive control.
Ciprofloxacin (or “cipro”) is a wide-spectrum antibiotic that kills many different types of bacteria. Although most students find that their oral bacteria is sensitive to ciprofloxacin, a few students each semester learn that they have ciprofloxacin-resistant bacteria in their
BIO 2 Lab Manual, Fall 2008 Version 9/14/08 Lab 4, Page 1
mouths. In a Kirby-Bauer assay, cipro-resistant bacteria grow up all over the plate and a zone of inhibition is not seen around the ciprofloxacin disc.
Antibiotics are agents that kill bacteria. The first antibiotic that was used to treat human disease was a class of agents called sulfa drugs. They were developed between the First and
Second World Wars by the German dye industry and revolutionized medicine. Before their development, bacterial infections were an extremely common cause of death. In the Civil War and in World War I, many more lives were lost to bacterial infections that set in after trauma due to bayonet or gunshot wounds than to the wounds themselves. While he was in office,
President Calvin Coolidge lost his son (Calvin Coolidge, Jr.) due to a bacterial infection that spread to his blood from a blister that he got on his foot after playing a game of barefoot tennis. Before the advent of antibiotics, such stories were all too common and parents lived in constant fear of losing their children to such seemingly innocuous injuries.
The first mass application of sulfa drugs was after the Japanese attack on Pearl Harbor on
December 7 th , 1941. Many American lives were saved because their injuries were packed with sulfa drugs and they did not suffer from post-traumatic infections of their wounds.
Antibiotics work by targeting specific properties of bacterial cells that are not shared by eukaryotic cells. If they killed human (eukaryotic) cells as well as bacterial cells, they would be much too toxic for use.
Sulfa drugs work because they are chemically similar to PABA (para-amino benzoic acid), a chemical that some bacteria require in order to make folic acid. Folic acid is needed to synthesize nucleic acids (DNA and RNA) and is therefore essential for cell function. When sulfa drugs are present, bacteria will pick it up instead of PABA. However, PABA cannot be used to make folic acid and the bacteria are therefore unable to make new DNA and cannot replicate their DNA and divide. This mechanism was not appreciated when sulfa drugs were first developed. At that time, no one knew how they worked. Since that time, scientists have discovered its molecular action.
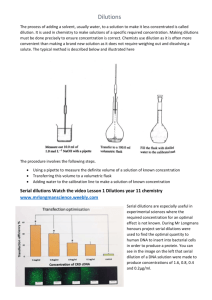
B. SERIAL DILUTIONS
Sometimes in the laboratory, a culture or reagent needs to be diluted by a large amount. This is often achieved by performing a series of serial dilutions rather than by one single (and very large) dilution.
BIO 2 Lab Manual, Fall 2008 Version 9/14/08 Lab 4, Page 2
For example, suppose you have a stock solution of a reagent with a concentration of 5 M
(mol/L) and you need to make 1 mL of a working concentration of only 0.005 M (= 5 mM).
This amounts to a total dilution of 1:1000. (5/0.005 = 1000)
You could achieve this by adding 1 mL of the stock solution into 999 mL of water, but this would make a full liter of the solution, and you don’t need anywhere near that much. Making up a full liter when you need only 1 mL would be wasteful, and can be expensive if the reagent costs a great deal.
Instead, you could set up a series of tubes, each containing 0.9 mL of water. Then you could add 0.1 mL (= 100 uL) of the stock solution to the first tube, to create 1 mL of a 1:10 dilution.
You could follow this by taking 0.1 mL of the 1:10 dilution and adding it to 0.9 mL of water to create another dilution, now at 1:100. By taking 0.1 mL of the 1:100 dilution and adding it to
0.9 mL of water, you would have exactly 1 mL of 1 1:1000 dilution (i.e. 0.005 M). This series of dilutions is called a serial dilution.
Note that if you chose the first method, you would need a total of 999 mL of water and 1 mL of your stock solution. By choosing the serial dilution method, you only need 27 mL of water and 0.1 mL of your stock solution. Obviously, the serial dilution method is more efficient and leads to less waste in the laboratory.
In today’s lab, you will set up a serial dilution of an overnight culture of E. coli cells. Usually, an overnight culture of E. coli contains about 10 9 cells per mL, but the number can vary. By serially diluting the culture and then plating samples of each dilution, your aim is to achieve a
countable and representative number of colonies on one of your plates. You can then use that plate to calculate the exact number of cells per mL in the original (undiluted culture). To be countable, a plate should contain less than about 300 colonies. To be representative (reliable), it should contain at least 30 colonies. (Why?) So your aim is to produce a plate with between
30 and 300 colonies.
In order to figure out how to set up your serial dilution, you need a little bit more information.
First, the plates we will be using are about 9 cm in diameter. Plates this size can comfortably accommodate about 0.1 mL of fluid for plating. (You can plate > 0.1 mL but the plates have to sit for a while to “soak up” the liquid before you turn them upside down for incubation.) So for this lab, you will plate exactly 0.1 mL of culture onto each plate. Second, the tubes you will be using for setting up your serial dilutions can accommodate up to 10 mL of water. So you will need to plan your serial dilutions such that the total volume in each tube never exceeds 10 mL. Third, you will be provided only 3 tubes of sterile water and 4 plates for your experiment.
BIO 2 Lab Manual, Fall 2008 Version 9/14/08 Lab 4, Page 3
You will plate undiluted culture onto one of the plates, so this leaves only 3 plates for plating your dilutions.
Before you come to lab, devise a plan for your serial dilutions and write up your plan
in your lab notebook. This is an excellent mental exercise and will help you understand the concept. Remember that your aim is to get between 30 and 300 colonies on one of the plates and that you must design your experiment within the limitations described above. In next week’s lab, you’ll have time to share your design with your lab partners and revise it, if needed. The main thing is to try to work through the problem on your own prior to coming to lab so you can find out where you are confused or need help.
III. Things to Do
3.
PART A. ANALYSIS OF RESULTS FROM MOUTHWASH EXPERIMENT
1. Retrieve your Kirby-Bauer test plate from the front bench.
Using a ruler, measure the diameter (in mm) of the growth-inhibition zone around each of the disks on your Kirby-Bauer test plate. If there is not a complete circle for the inhibition zone, measure the radius in mm and multiply by 2.
2. Record your results in your lab notebook, using the following table format:
Disk
Ciprofloxacin (+ control)
Water (- control)
Scope
Listerine
Diameter (mm)
Assuming that the size of the growth-inhibition zone directly relates to the effectiveness of the product in inhibiting the growth of oral bacteria, discuss the following questions with your lab partners and write the answers in your lab notebook. a.
Write out a hypothesis for this experiment. Make sure you have at least one independent and one dependent variable. Which is which? b.
Did your positive control work? Explain. c.
Did your negative control work? Explain.
BIO 2 Lab Manual, Fall 2008 Version 9/14/08 Lab 4, Page 4
d.
Was either of the mouthwashes more effective than water at inhibiting the growth of oral bacteria? e.
If both products were more effective than water, which was better? (If any?) f.
Does your data support or disprove your hypothesis? Explain. g. Are the results obtained by your group consistent with those obtained by other
groups in the lab? Why might it be important to find out?
PART B. INTERPRETATION OF RESULTS FROM EMB PLATING
1.
Retrieve your EMB agar plate from the 37 degree incubator.
2.
Examine the plate carefully. Are there any colonies on the plate? If so, describe their morphologies and record your results in your lab notebook.
3.
Was the colony you chose from your environmental plate last time a coliform? If so, was it a non-E. coli coliform or is it E. coli? Use the plates of known coliforms and E. coli at the front of the lab to help you.
4.
Be sure that you understand how EMB agar works as both a selective and differential medium. If you don’t remember, review the introduction to last week's lab.
5.
When you’re done, discard your plates in the large biohazard bag at the front of the room. (Why?) After class, the bag will be taken to the autoclave room for sterilization.
PART C. SERIAL DILUTIONS OF AN OVERNIGHT CULTURE OF E. COLI
When you get back to the lab, obtain 3 blanks, each containing 9 mL of sterile water (why sterile?), and 4 EMB agar plates.
1.
Get out your serial dilution scheme and discuss it with your lab partners. Then we will discuss it as a group before proceeding.
2.
Set up your serial dilutions using sterile water and sterile micropipettor tips. (Why?)
3.
Label your plates (on the bottom) 1-4.
BIO 2 Lab Manual, Fall 2008 Version 9/14/08 Lab 4, Page 5
4.
Plate 0.1 mLs of undiluted solution onto plate 1. Then plate 0.1 mL of your serial dilutions onto plates 2-4. Plate 4 should have your most dilute solution.
5.
Tape your plates together and label the tape with the date, your lab section number, and your table number (or the initials of everyone in your group).
6.
Take your plates to the 37 degree incubator. The colonies will be allowed to grow up overnight and then your plates will be refrigerated (to prevent overgrowth) until the next lab period.
V. Lab Clean-Up
All Kirby-Bauer and EMB plates should be discarded in the large biohazard bag at the front of the class.
Gloves can be discarded in the regular trash (unless you touched bacteria with them, in which case they should be discarded in the large biohazard bag at the front of the room.)
Wash your hands before leaving lab.
BIO 2 Lab Manual, Fall 2008 Version 9/14/08 Lab 4, Page 6