File
advertisement
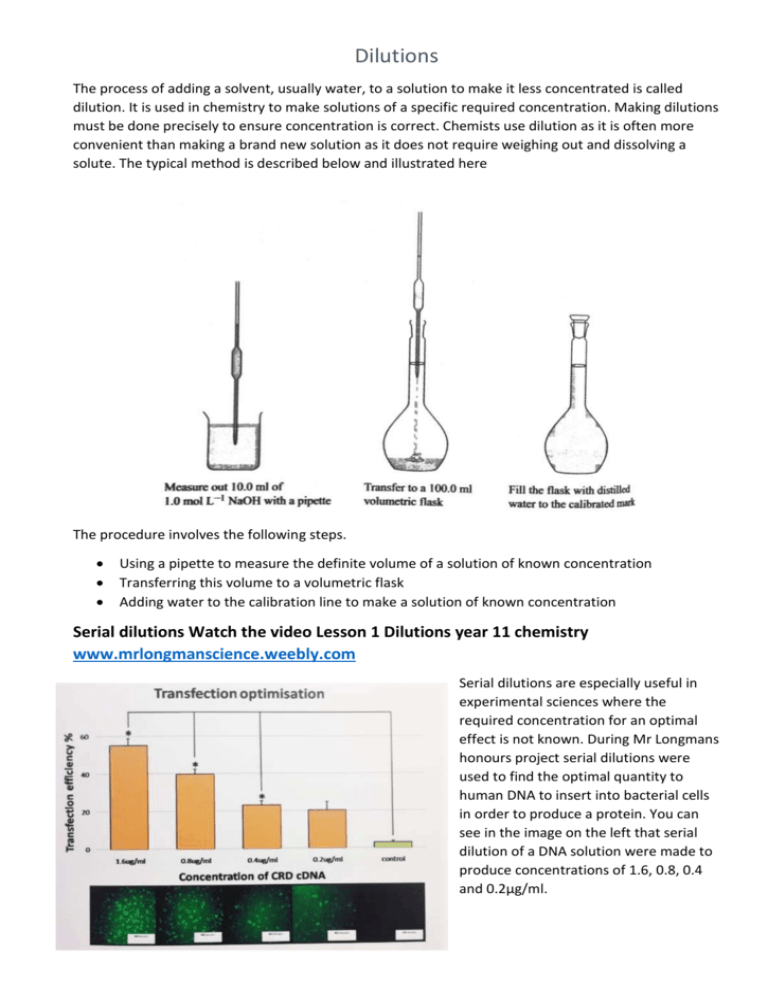
Dilutions The process of adding a solvent, usually water, to a solution to make it less concentrated is called dilution. It is used in chemistry to make solutions of a specific required concentration. Making dilutions must be done precisely to ensure concentration is correct. Chemists use dilution as it is often more convenient than making a brand new solution as it does not require weighing out and dissolving a solute. The typical method is described below and illustrated here The procedure involves the following steps. Using a pipette to measure the definite volume of a solution of known concentration Transferring this volume to a volumetric flask Adding water to the calibration line to make a solution of known concentration Serial dilutions Watch the video Lesson 1 Dilutions year 11 chemistry www.mrlongmanscience.weebly.com Serial dilutions are especially useful in experimental sciences where the required concentration for an optimal effect is not known. During Mr Longmans honours project serial dilutions were used to find the optimal quantity to human DNA to insert into bacterial cells in order to produce a protein. You can see in the image on the left that serial dilution of a DNA solution were made to produce concentrations of 1.6, 0.8, 0.4 and 0.2µg/ml. Calculations for Dilutions As the number of moles of solute remains the same throughout we can use the Dilution formula to obtain the final concentration of the solution. Dilution formula c1 V1 = c2 V2 Examples: Refer to your text Page 318 – worked example 7.4 for a worked example of using the Dilution formula Another more complex example if given here. Example question: 10ml of an 18M solution of sulfuric acid was diluted with 140ml of water to make a dilute sulfuric acid mixture. This new sulfuric acid solution was then reacted to completion with a 10M sodium hydroxide solution. What was the volume of the sodium hydroxide solution? H2SO4 + NaOH NaHSO4 + H2O H+ + OH- H2O Step 1. Find the concentration of final solution of H2SO4 (C2) c1V1 = c2V2 18 x 0.01 = c2 0.150 c2 = 1.20M Step 2. Calculate moles of H2SO4 n= cxV n = 1.20 x 0.150 n = 0.18 mol Step 3 use reaction equation to find reaction ratio 1 H2SO4 : 1 NaOH 0.18mol (H2SO4) : 0.18 mol (NaOH) Step 4 Volume of the NaOH Solution n (NaOH) = c x V 0.18 = 10 x V V = 0.0180 L 18.0 ml