Heat Treatment and Mechanical Properties of Carbon Steel
advertisement

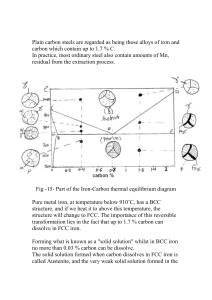
Carbon Steels: Microstructure and Mechanical Properties Reading Assignment: 9.14, chapter 10 in Callister Objectives • Recognize the wide range of equilibrium microstructures and the effect of C content on the mechanical properties. • Recognize the main heat treatments for steels and the corresponding mechanical properties. Introduction Steel is one of the most used engineering materials. It is used in the form of beams for building support structures, train railroads, and reinforcing rods in concrete; in the form of plates for ship construction; in the form of tubes for boilers in power generating plants, car radiators, and oil and gas pipelines; in the form of sheet metal for cars, washing machines, in the form of wire for elevator cables, and special steels are used for cutting tools (hacksaw, blades, drill bits, knives) and for wear resistant application such as ball bearings. There are two main reasons for the popular use of steels: (1) steel is abundant in the earth’s crust in the form of Fe2O3 and require little energy to convert it to Fe which makes its production inexpensive; and (2) it can be made to exhibit a great variety of microstructures and thus a wide range of mechanical properties. The microstructure that develops in carbon steels depends on both (1) the carbon content and (2) thermal history or heat treatment. Equilibrium Phases To understand the microstructures that can be produced by heat treatment of steel, it is necessary to consider the Fe-C phase diagram (Fig. 1). There are three equilibrium phases in the phase diagram which can be obtained by very slow cooling rates to allow equilibrium conditions to prevail. Each phase has particular characteristics, some of which are listed in Table 1. eutectoid Figure 1: phase diagram for Fe-C showing the range of carbon steels. 1 Table 1 Characteristics of the Equilibrium Phases in Steel phase crystal composition strength ductility Structure solid solution 0 to 2.1 wt% C Low High Austenite (γ) of C in FCC Fe Ferrite (α) Cementite (Fe3C) Solid solution of C in BCC Fe Orthorhombic compound Intermediate Intermediate Extremely hard Extremely Brittle 0 to 0.02 wt% C 6.7 wt% C The amount of equilibrium phase changes that take place upon slow cooling from the austenite region in the Fe-C phase diagram into the ferrite + cementite phase field strongly depends on the carbon content. Depending on the carbon content, carbon steels can be divided into three categories: eutectoid steels (contain exactly 0.76%C), hypoeutectoid steels (%C < 0.76), and hypereutectoid steels (%C> 0.76). The microstructure that develops when a eutectoid steel (0.76% C) is slowly cooled from the austenite region to below 727 ºC consists of alternating layers of α and cementite. This structure is called pearlite. For hypoeutectoid steels (%C < 0.76) the microstructure consists of pearlite surrounded by pro-eutectoid α while hypereutectoid steels (%C> 0.76) are composed of pearlite surrounded by cementite, as illustrated in Figure 1. The equilibrium amounts of ferrite and cementite can be calculated by the use the lever rule. The hardness of carbon steels increases with increasing the carbon content due to increases in the hard phase, cementite. It should be noted that slow cooling heat treatment is not important from practical point of view. It is used here just to demonstrate the objectives of this experiment. Refer to your textbook for further details. Experimental Procedure Part#1 1. You are given 4 mounted specimens of carbon steels i. Sp#1 with 0.4%C Low carbon steel ii. Sp#2 with 0.6%C (AISI 1040) Medium carbon steel iii. Sp#4 with 0.8%C (AISI 1080) Eutectoid steel iv. Sp#5 with 1.1%C High carbon steel 2. Fully austenize the specimens by heating at 850 ºC for 20 minutes in a heat treatment furnace. Austenitizing refers to heating the steel into the austenite phase field so that all of the carbon is dissolved into solid solution γ. 3. Cool the specimens inside the furnace at very slow rate to get the equilibrium structures (decrease the furnace temperature from 850 ºC to 720 ºC and then quench in water to retain the microstructure). 4. Take five measurements of the hardness of each sample using the Rockwell hardness scale B. 5. Etch the specimens using 2% Nital. 6. Observe the microstructure in the optical microscope at 100X. 7. Take photos for the microstructures. 2 8. Calculate the fractions of total ferrite and cementite for each specimen from the phase diagram and the lever rule. 9. Calculate the fractions of the proeutectoid phase and pearlite for each alloy. 10. Prepare your data in a table form as shown below. 11. Plot the average hardness, total ferrite, and total cementite vs. C%. 12. Which phase is responsible for the increase in hardness? C% 0.2 0.4 0.8 1.2 Total ferrite Total cementite Hardness Non-equilibrium Heat treatments of Steels From the previous experiment, you have looked at the effect of C on the strength for different steels cooled from the austenizing temperature very slowly to reach equilibrium conditions. In practice, however, it is not practical to cool at very slow rate to get the equilibrium microstructure and real heat treatments almost always involve the development of non-equilibrium microstructures. Note that the phase diagram can not be used to predict the non-equilibrium microstructures. Heat treatments of steels will be divided into two approaches: intermediate cooling or fast cooling as explained below. Intermediate cooling: when the steel is cooled at intermediate rates to room temperature, C can diffuse relatively far and the spacing of the C rich phase Fe3C is greater. The resulting pearlite is called coarse pearlite and the heat treatment is called full anneal. This is can be done by shutting off the furnace while the specimen is kept inside. When the steel is cooled at a faster rate (but still slower than quenching), the transformation takes place at temperatures quite a bit below 727oC. At the lower temperature C can diffuse only a short distance, and the spacing of the C rich phase Fe3C is smaller. The resulting pearlite is called fine pearlite and the heat treatment is called normalizing. This is can be done by taking the specimen from the furnace and let it cool at room temperature. The range of lamellar spacings in steels vary from about 1 µm to 0.1 µm. Figure 2: (a) coarse pearlite resulting from full anneal and (b) fine pearlite from normalizing. (a) (b) 3 Fast cooling or quenching: the previous section provided an indication that the rate of cooling has an effect on the microstructure of steel. This is because the C atoms are in solution in the FCC austenite phase. When the austenite is slowly cooled and the crystal structure of Fe changes to bcc, the phase diagram shows that most of the C atoms must diffuse to form particles of Fe3C, leaving only a small concentration of C atoms dissolved in the BCC Fe crystal. Suppose that we cool the austenite very quickly - so fast that the C atoms don't have time to diffuse before the steel is cooled to such a low temperature that the C atoms are frozen in position. The Fe atoms only have to shift their Figure 3: martensite positions slightly to accomplish the transformation from fcc austenite to bcc ferrite; therefore, fast cooling does not stop the austenite to ferrite transformation from taking place. What it does stop is the diffusion of C atoms over the distance necessary for them to form Fe3C particles. Thus, we have a different situation than anything indicated on the Fe-C phase diagram: ferrite containing very high %C in solid solution. The C trapped in solid solution in BCC Fe stretches the lattice in only one direction, so that the unit cell no longer has 3 sides of equal length. For this reason, the quenched steel containing extra C in BCC Fe is usually said to have a body centered tetragonal (BCT) crystal structure. In an optical microscope, the individual atoms are not resolved so this aspect of the structure is not visible. What can be seen are the feathery (lath-shaped) crystals of Fe that contain more C in solid solution than predicted by the phase diagram. Fig. 3 shows a microstructure of quenched 1080 steel; this structure is know as martensite. The elongated lath shape of the martensite crystals is a consequence of the way the transformation from austenite (FCC) to ferrite (BCC) takes place. Martensite is the strongest and the most brittle phase in steel. Experimental Procedure Part#2 1. You are given 6 specimens of eutectoid steels (0.8%C) 2. Fully austenize the specimens by heating at 750 ºC for 20 minutes in a heat treatment furnace. 3. Cool two specimens inside the furnace to room temperature (shut off the furnace). This is called full anneal. 4. Cool two specimens at room temperature. This is called normalizing. 5. Quench two specimens in tap water. 6. Take five measurements of the hardness of each sample using the Rockwell hardness scale C. Full anneal Normalizing Figure 4: continuous cooling curve for eutectoid steel. 4 7. Etch the specimens using 2% Nital. 8. Observe the microstructure in the optical microscope at 100X. 9. Take photos for the microstructures. 10. Prepare your data in a table form as shown below. 11. Rank the heat treatments in a decreasing order of hardness. The non-equilibrium microstructure can be predicted transformation diagram as demonstrated in Figure 4. Heat treatment Slow cooling (Full anneal) Intermediate (Normalizing) Fast cooling (Quenching) Structure Coarse pearlite Fine pearlite martensite Hardness by the continuous Ductility 5