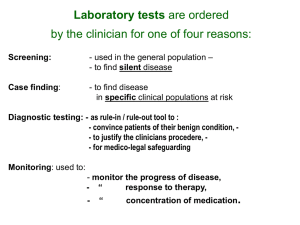
course syllabus - UCSD Lab Medicine
advertisement

UNIVERSITY OF CALIFORNIA SAN DIEGO SKAGGS SCHOOL OF PHARMACY AND PHARMACEUTICAL SCIENCES SPPS 216 CORE COURSE IN LABORATORY MEDICINE Winter 2011 Robert L. Fitzgerald, Ph.D., DABCC Course Chairman UNIVERSITY OF CALIFORNIA SAN DIEGO SKAGGS SCHOOL OF PHARMACY AND PHARMACEUTICAL SCIENCES COURSE MANUAL SPPS 216 CORE COURSE IN LABORATORY MEDICINE Winter 2011 Robert L. Fitzgerald, Ph.D., DABCC Course Chairman ACKNOWLEDGEMENTS The following individuals are acknowledged for their contributions to the course and this manual: All of the individuals who contributed to the SOM 216 Laboratory Medicine manual, which this manual is adapted from, are gratefully acknowledged. Over the last twenty years, the SOM 216 manual has developed into an outstanding collection of practical clinical information contributed by many professionals. The more recent version of the SOM 216 manual primarily reflects the work of three individuals; Dr. Norbert Tietz, Dr. David Herold, and Dr. David Bailey. In addition to contributing to the SOM 216 manual, Dr Bailey read this entire manual and his input was invaluable. Dr. Stephen Baird’s input in many areas was also essential, especially the chapters involving hematology. i ii Fast is fine, but accuracy is everything. - Wyatt Earp (1849-1929), American gambler, gunfighter, and lawman iii iv FOREWORD Laboratory medicine provides objective data before, during, and after deciding proper course of therapy. Throughout your career as a pharmacist, you will be required to interpret laboratory data in order to optimize drug therapy. This manual is designed to be an introduction to that process. Chapters are organized to show objectives, define key terms, provide background, and then highlight important topics within that section. At the end of most chapters case reports highlight the importance of interpreting laboratory results in the context of clinical therapeutics. Hopefully, as you develop professionally, you will continue to consult with laboratory professionals. This is especially important when the laboratory data does not appear to match the patient’s clinical picture. As Frank Herbert said, “The beginning of knowledge is the discovery of something we do not understand.” Robert L. Fitzgerald, Ph.D. Course Chairman, 2011 v vi TABLE OF CONTENTS COURSE SCHEDULE 1 SELECTED REFERENCES FOR OPTIONAL READING 2 ADJUNCT SOURCES OF INFORMATION 2 STUDENT EVALUATION 3 COURSE AND FACULTY EVALUATIONS 3 REFERENCE RANGES AND CONVERSION FACTORS TABLE 4 I. II. CLINICAL LABORATORY STATISTICS 7 LABORATORY DIAGNOSIS OF CARDIAC DISEASE 19 III. LABORATORY DIAGNOSIS OF DYSLIPIDEMIA 31 IV. LABORATORY DIAGNOSIS OF RENAL DISEASE 41 LABORATORY TESTS OF GASTROINTESTINAL DISEASE 57 LABORATORY DIAGNOSIS OF PANCREATIC DISEASE 69 LABORATORY DIAGNOSIS OF PROTEIN ABNORMALITIES 77 LABORATORY DIAGNOSIS OF DIABETES MELLITUS 91 V. VI. VII. VIII. IX. ELECTROLYTES AND ACID-BASE BALANCE 105 LABORATORY DIAGNOSIS OF ADRENOCORTICAL DISEASE 129 LABORATORY DIAGNOSIS OF THYROID DISEASE 147 LABORATORY DIAGNOSIS OF LIVER DISEASE 165 XIII. TUMOR MARKERS 177 XIV. PREGNANCY & PRENATAL ANALYSIS 183 LABORATORY DIAGNOSIS OF IRON AND RED BLOOD CELL DISORDERS 193 HEMOSTASIS AND WHITE BLOOD CELL DISORDERS 203 CLINICAL TOXICOLOGY 215 THERAPEUTIC DRUG MONITORING 233 X. XI. XII. XV. XVI. XVII. XVIII. GLOSSARY 247 vii viii COURSE SCHEDULE – WINTER 2011 The course meets in PSB 1120. Each session is 2 hours with a 10 min break. Students are expected to read the syllabus chapters and work on the cases prior to each session. Session # Date Time 1 Tuesday 1/4/11 1:002:50 2 Tuesday 1/11/11 1:002:50 3 Tuesday 1/18/11 1:002:50 Tuesday 1/25/11 Friday 1/28/11 6 Tuesday 2/8/11 1:002:50 1:002:50 1:002:30 2:302:50 1:002:50 7 Tuesday 2/15/11 1:002:50 Tuesday 2/22/11 1:002:50 Tuesday 3/1/11 Friday 3/4/11 1:002:50 1:002:50 1:002:30 2:302:50 4 5 8 9 10 Tuesday 2/1/11 Tuesday 3/8/10 Topic (& Required Reading) Clinical Laboratory Statistics (Chapter I) Laboratory Diagnosis of Cardiac Disease (Chapter II) Laboratory Diagnosis of Dyslipidemia (Chapter III) Laboratory Diagnosis of Renal Disease (Chapter IV) Laboratory Tests of Gastrointestinal Disease (Chapter V) Laboratory Diagnosis of Pancreatic Disease (Chapter VI) Laboratory Diagnosis of Protein Abnormalities (Chapter VII) Laboratory Diagnosis of Diabetes Mellitus (Chapter VIII) Electrolytes and Acid-Base Balance (Chapter IX) Lecturer Fitzgerald Fitzgerald Herold Fitzgerald Fitzgerald Q&A Session Exam I Optional Exam Review Laboratory Diagnosis of Liver Disease (Chapter XII) Hemostasis and White Blood Cells (Chapter XVI) Laboratory Diagnosis of Adrenocortical Disease (Chapter X) Tumor Markers (Chapter XIII) Pregnancy & Prenatal Analysis (Chapter XIV) Laboratory Diagnosis of Iron and Red Blood Cells (Chapter XV) Baird Baird Laboratory Diagnosis of Thyroid Disease (Chapter XI) Herold Clinical Toxicology (Chapter XVII) Therapeutic Drug Monitoring (Chapter XVIII) Fitzgerald Fitzgerald Fitzgerald Fitzgerald Q&A Session Exam II Optional Exam Review 1 SELECTED REFERENCES FOR OPTIONAL READING 1. Burtis CA, Ashwood ER and Bruns DE (eds), Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 4th edition, St. Louis: Elsevier Saunders, 2006. 2. Kaplin LA, Pesce AJ and Kazmierczak SC, Clinical Chemistry Theory, Analysis, Correlation, 5th edition, St. Louis: Mosby, 2009. 3. Koda-Kimble MA et al. (eds), Applied Therapeutics: The Clinical Use of Drugs, 9th edition, Philadelphia: Lippincott Williams and Wilkins, 2008. 4. McClatchey KD (ed), Clinical Laboratory Medicine, 2nd edition, Philadelphia: Lippincott Williams & Wilkins, 2002. 5. Sacher RA and McPherson RA, Widmann’s Clinical Interpretation of Laboratory Tests, 11th edition, Philadelphia: Davis, 2000. 6. Wu AHB (ed), Tietz Clinical Guide to Laboratory Tests, 4th edition, St. Louis: Saunders/Elsevier, 2006. ADJUNCT SOURCES OF INFORMATION Course Web Site (WebCT) <http://webct.ucsd.edu>: Lecture PPT’s will be posted here as well as the complete course manual. 2 STUDENT EVALUATION The course will have two exams. Each exam will have 300 points possible, and will consist of two parts: part 1 (200 points) will consist of 40 multiple choice questions; part 2 (100 points) will be more practical and case-based. Thus, the total number of points possible from both exams is 600. A passing score is 75% (450/600), and honors will be given for a total score greater than 93%. Each chapter will be equally represented in the exams. If a student fails to achieve an overall score of 75% (450/600), then the student fails the course. At the discretion of the Course Director, and in consultation with the School of Pharmacy, an oral makeup exam may be given. In order to qualify for the oral exam, the student needs to have attended lecture sessions and must be performing adequately in other required SPPS/SOM courses. If given, the oral exam typically lasts two hours and is comprehensive for the entire course. Typically the exam will be administered by three pathology faculty members, one of which will be the SPPS 216 Course Director. The oral exam is pass/fail. The general policy of this course will be consistent with SPPS/SOM policy with regards to missed exams. The only excuse accepted for missing exams will be related to personal health, family health, or professional activities. Do not ask to miss an exam to go to a graduation, wedding, etc. Although there is no official honor code at UCSD, this course is operated using an honor code. Exams are closed book and no other supplemental material, computers, cell phones, or other electronic aids may be used during the exam. Calculators will be provided at the exams. It is the student’s responsibility to ensure that all work is their own and that no other student has copied their work. All exams will be proctored and any student found cheating will fail the course and be subject to review by the SPPS. COURSE AND FACULTY EVALUATIONS Course and faculty evaluations provide important feedback to instructors to improve course content and teaching methodology. Teaching evaluations are also an important factor in faculty advancement, merit and promotion. The School and the University require this information. As such, completion of course and instructor evaluations is a requirement for successful completion of this course. This is also part of developing professional conduct and behavior. To facilitate ease of completion of evaluations an electronic format has been implemented. (Please see the SPPS website for the link). Students who have academically passed this course but who have not completed: 1) the evaluation of the course, 2) the evaluation of the course chair(s), and 3) evaluations of at least 90% of guest lecturers, will receive a grade of “I” (Incomplete) for the course. If an “I” grade has been assigned due to incomplete evaluations, changing the “I” grade, will require completion of the evaluations prior to the start of the next academic quarter. A grade of “I” must be changed to an “H” (Honors) or “P” (Pass) by the end of the next academic quarter in which the student is enrolled or the grade is automatically changed by the Registrar’s Office to an “F” (Fail). A petition is required to change the “I” grade. 3 REFERENCE RANGES AND CONVERSION FACTORS Reference ranges are valuable guides for the clinician, but they should not be regarded as absolute indicators of health and disease. Reference ranges should be used with caution, since values for healthy individuals often overlap significantly with values for persons afflicted with disease. In addition, laboratory values may vary significantly because of differences in methodology and mode of standardization. This is especially true for immunological tests, which use antibodies that may have different characteristics. As a result, laboratory values in individual institutions may differ from those listed in this manual. Analyte Range Serum ACTH Albumin 7-50 3.4-4.8 Supine: < 1.6-16 Upright: 4.0-31 30-130 11-45 28-85 10-35 10-30 SI Units 1 10 ng/dL 0.0277 U/L U/L U/L mol/L U/L 1 1 1 1 1 ng/L g/L nmol/L nmol/L U/L U/L U/L mol/L U/L 22-28 Total < 1.2 Conjugated < 0.2 < 100 mEq/L 1 mmol/L mg/dL 17.1 mol/L 0.9-3.9 < 0.3 8.5-10.4 95-107 140-220 24-31 35-48 Male: 70-140 Female: 88-155 Morning: 7-25 Evening: < 9 0-178 < 6% of total <7 < 2.5 0.4-1.2 ng/mL mg/dL mg/dL mEq/L mg/dL mmol/L mmHg 0.33 10 0.25 1 0.026 1 1 nmol/L mg/L mmol/L mmol/L mmol/L mmol/L mmHg g/dL 0.16 mol/L g/dL 27.6 27.6 1 1,800-7,700 x 103 200-2,100 x 103 1,500-6,700 x 103 0-700 x 103 0-150 x 103 1,500-4,000 x 103 200-950 x 102 cells/L cells/L cells/L cells/L cells/L cells/L cells/L Male: 4.3-5.7 x 106 Female: 4.2-5.7 x 106 cells/L Alkaline Phosphatase ALT Amylase Ammonia AST Bicarbonate Bilirubin BNP C-Peptide C-Reactive Protein Calcium Chloride Cholesterol CO2, total pCO2 (WB, arterial) Copper Cortisol Creatine Kinase (CK) Creatine Kinase (MB) CK Index Creatinine Differential Leukocyte Counts Neutrophils, total Bands Segmented Eosinophils Basophils Lymphocytes Monocytes 4 Conversion (mult. by) pg/mL g/dL Aldosterone Erythrocyte Count Red Cell Count Conventional Units pg/ml U/L nmol/L U/L ng/mL mg/dL 88.4 mol/L REFERENCE RANGES AND CONVERSION FACTORS Analyte Ferritin Folate Glucose HDL - cholesterol Hematocrit Hemoglobin Hemoglobin A1c Range Male: 30-400 Female: 10-120 4-20 70-99 Male: > 35 Female: > 45 Male: 40-50 Female: 37-47 Male: 14-18 Female: 12-16 3.8-6.4 Conventional Units Conversion (mult. by) SI Units ng/mL 1 g/L ng/mL 2.26 nmol/L mg/dL 0.055 mmol/L mg/dL 0.026 mmol/L % vol. g/dL % 228-428 5-35 Male: 65-170 Female: 50-170 g/dL U/mL 0.18 6.95 mol/L pmol/L g/dL 0.18 mol/L 6.4-16.5 25-100 < 10 mg/dL U/L g/dL 0.11 1 0.048 mmol/L U/L mol/L 4.5-11.0 x 103 cells/L 23-300 0.5-1.5 U/L mEq/L 1 1.0 U/L mmol/L Magnesium Myoglobin 1.7-2.6 < 100 mg/dL g/L 0.41 mmol/L Osmolality 270-310 mOsm/kg 1 mOsm/kg 35-45 85-108 7.35-7.45 2.5-4.5 150-450 x 103 3.5-5.0 6.0-8.0 mmHg mmHg 1 1 mmHg mmHg mg/dL cells/L mEq/L g/dL 0.32 mmol/L 1 10 mmol/L g/L 135-145 mEq/L 1 mmol/L ng/dL 3.47 nmol/L g/dL ng/dL 12.9 12.9 nmol/L nmol/L mg/dL 0.01 g/L mg/dL ng/dL 0.0113 0.0154 mmol/L mmol/L ng/mL n/a n/a U/mL 1 mU/L mg/dL 0.36 mmol/L mg/dL 59.48 mol/L IBC (total) Insulin Iron Lactate LD Lead Leukocyte count White Cell Count Lipase Lithium pCO2 (WB, arterial) pO2 (WB, arterial) pH (WB, arterial) Phosphate Platelet count Potassium Protein (Total) Sodium Testosterone Thyroxine (total T4) (free T4) Transferrin Triglycerides Triiodothyronine (T3) Troponin Cardiac Troponin I (cTnI) Cardiac Troponin T (cTnT) TSH Urea (urea nitrogen) Urate (uric acid) Male: 185-650 Female: 10-45 4.5-10.5 0.7-1.9 Male: 187-318 Female: 218-330 30-200 60-181 < 0.07 < 0.1 0.49-4.67 8-23 Male: 3.6-7.5 Female: 3.6-6.4 5 REFERENCE RANGES AND CONVERSION FACTORS Analyte Vitamin B12 Vitamin D 25 OH-D 1,25(OH)2-D Zinc Urine Aldosterone (dependent on Na intake) Calcium (diet dependent) Catecholamines: Epinephrine Norepinephrine Cortisol, free Creatinine Creatinine clearance 5 HIAA Metanephrine Oxalate Phosphate Specific Gravity Urea (urea nitrogen) Conventional Units Range Conversion (mult. by) SI Units 160-1060 pg/mL 0.738 pmol/L 15-57 15-50 ng/mL pg/mL 2.5 2.4 mmol/L pmol/L Male: 75-291 Female: 65-256 g/dL 0.1530 mol/L 6-25 g/24h 2.77 nmol/d 100-250 mg/24h 0.025 mmol/d 0-25 0-100 20-90 Male: 1-2 Female: 1-1.8 90-125 3-15 30-350 Male: 7-44 Female: 4-31 0.3-1.0 1.010-1.025 10-20 g/24h g/24h g/24h 5.46 5.91 2.76 nmol/d nmol/d nmol/d g/24h 8.84 mmol/d mL/min mg/24h g/24h g/24h 1.0 5.2 5.458 1.4 11.4 32.3 mL/min mol/d nmol/d mol/L mol/L mmol/L g/24h 35.7 mmol/d mg/24h Miscellaneous Blood Pressure Heart Rate Respiration Rate < 140 Diastolic < 90 6 bpm (beats per minute) min 50-100 16 Blood Cell Values in a Normal Population White cell count, 103/μl blood Red cell count, 106/μl blood Hemoglobin, g/dL blood Hematocrit, % Mean corpuscular volume, fl/red cell Mean corpuscular hemoglobin, pg/red cell Mean corpuscular hemoglobin concentration, g/dL RBC Red cell distribution width, CV (%) Platelet count, 103/μL blood Erythrocyte sedimentation rate (ESR), mm/hr Serum viscosity Metric conversions 1 meter (m) = 39.4 inches 1 cm = 0.394 inches 1 kg = 2.2 lbs mm mean (reference range) Women 7.8 (4.4-11.3) 5.21 (4.52-5.90) 4.60 (4.10-5.10) 15.7 (14.0-17.5) 13.8 (12.3-15.3) 46 (42-50) 40 (36-45) 88 (80.0-96.1) 30.4 (27.5-33.2) Men 34.4 (33.4-35.5) 13.1 (11.5-14.5) 311 (172-450) 0-10 0-20 1.1-2.0 I CLINICAL LABORATORY STATISTICS 7 8 A. OBJECTIVES To understand how reference ranges are determined and how to use them to interpret laboratory data To understand common statistical terms related to laboratory diagnostics To understand how disease prevalence affects predictive values of laboratory tests To understand the basic principle of quality control as applied to a diagnostic test B. KEY TERMS Accuracy - closeness of agreement of a measured value and the true value Analytical variation - observed differences in the value of an analyte after it has been prepared for analysis Coefficient of variation - a relative measure of precision, determined by dividing the standard deviation by the mean Intraindividual variation - differences in true value of an analyte within the same individual Interindividual variation - differences in true value of analyte between individuals Mean - the arithmetic average Negative predictive value - the fraction of negative values which are correct; determined by dividing the true negatives by the sum of the true negatives and false negatives Positive predictive value - the fraction of positive values which are correct; determined by dividing the true positives by the sum of the true positives and false positives Precision - the closeness of agreement between independent measurements; generally expressed as coefficient of variation or standard deviation Reference range - the inner 95% of values for a laboratory test as measured in a defined population; the subject population is typically disease free with regards to the test of interest Sensitivity - the ability of a test to detect a true positive; determined by dividing the true positives by the sum of the true positives and false negatives Specificity - the ability of a test to detect a true negative; determined by dividing the true negatives by the sum of the true negatives and false positives Standard deviation - a measure of precision (square root of the variance) 9 C. BACKGROUND/SIGNIFICANCE A basic understanding of statistics is assumed for this chapter. In order to interpret laboratory tests it is essential that pharmacists understand sources of variation, reference ranges, predictive values and how laboratory results are interpreted. D. SOURCES OF VARIATION Proper interpretation of results requires an understanding of the sources of variation which influence laboratory tests. Analytical variation is produced by conditions which affect the sample and the testing system from the moment the sample is removed from the patient until the final result is generated. (It is helpful to further subdivide this category into preanalytical factors, which include all the things that can happen to a sample as it is collected, transported, processed, and stored, and analytical factors which affect the testing process itself.) All test results are subject to analytical variation. Important interferences with laboratory tests include hemolysis (rupture of RBC into plasma), lipemia (excess lipids in a plasma sample) and icteric (high concentrations of bilirubin). Table 1: Hemolysis Interferences with Common Lab Tests Test Sodium, mEq/L Potassium, mEq/L Transaminase (ALT), U/L Folates, ng/dL Erythrocyte 16 100 500 200-1200 Plasma 140 4.4 25 2.5-1.5 Effect of hemolysis Lower plasma result Raises plasma result Raises plasma result Raises plasma result Table 2: Examples of Effects of Drugs on Laboratory Tests Test Bilirubin Bilirubin Amylase Digoxin Effect Decrease Increase Increase Increase Mechanism Barbiturates induce glucuronyl transferase Any drug with liver toxicity (e.g., acetaminophen) Opiates cause constriction of sphinter of Oddi Quinidine releases digoxin from heart muscle and decreases renal clearance causing substantial increases in serum digoxin Intraindividual variation is produced by conditions which cause a single individual’s laboratory values to change at different times of day or under different physiologic conditions. Examples of factors which contribute to intraindividual variation include circadian rhythms, hydration, activity, stress, posture, and food intake. When we use the results of serial testing to follow the course of disease in a patient, it is important to recognize the potential contribution of normal physiologic factors and try to distinguish it from medically important variation. Interindividual variation reflects the many different factors which cause laboratory test results to vary from one individual to another within a population. Examples of such 10 variables include age, sex, diet, body mass, general activity level, and genetics. The results of a test performed on a group of individuals will reflect analytical variation and intraindividual as well as interindividual variation. The remainder of this chapter addresses each of these categories in more detail, together with the applicable statistical concepts. Familiarity with the normal distribution (bell-shaped curve, or Gaussian distribution), including the concepts of the mean and standard deviation, is assumed. E. ESTABLISHING REFERENCE RANGES When we establish a reference range, we want to have a tool for comparing the test result from one individual with those from a relatively large number of other members of a similar population. What we want to determine is the expected range of interindividual variation. We already know that the results of a test performed on a group of people will reflect intraindividual and analytical variation as well as interindividual variation; it is obvious that if the contributions from the first two are relatively large, they will obscure the part of the total variation that is due to actual differences among individuals. Part of the process of establishing a reference range is simply taking steps to reduce the magnitude of this obscuring effect. Define the reference population. Demographically, it should match the population whose laboratory results will be compared to this reference range. Based on what is already known about the analyte, consider whether separate reference ranges should be established for adults versus children, men versus women, and so forth. Profound biochemical changes take place in the period between birth and adulthood, and many of these are reflected by clinical chemistry test values in this age group that differ significantly from those considered normal in adults. The most pronounced and/or accelerated changes are seen in the newborn period and during puberty. Table 3 gives examples of laboratory tests that are affected by age. Some hospitals only give reference ranges for adults, yet report out children’s results (with incorrect reference ranges), thus it is important to know what values change with age. Table 3: Lab Results that are affected by Age Lab Tests that are Higher in Newborns and Children Alkaline Phosphatase Ammonia AST Bilirubin Creatine Kinase (CK) Potassium Gamma glutamyl transferase (GGT) Thyroid stimulating hormone (TSH) Thyroxine (T4) Lab Tests that are Lower in Newborns and Children Bicarbonate Albumin Amylase Cholesterol Creatinine Copper Glucose Haptoglobin IgA, IgM, IgE Osmolality 11 If the distribution is Gaussian, a parametric method may be used. The reference range is defined as the mean plus or minus two standard deviations: Reference Range x 2SD . If the distribution is non-Gaussian, a non-parametric method must be used, but the reference range is still the inner 95% of the defined population. We have a specific term for the values of healthy individuals which fall outside the limits of the reference range: we call them “false positives” regardless of whether they fall at the high or low end of the range. The terms “positive” and “negative” in this context have nothing to do with high or low numbers, but rather indicate positivity versus negativity for disease. Healthy 95% 2.5% False Positives Reference Range 2.5% False Positives Figure 1: Graphic Representation of a Reference Range F. USING REFERENCE RANGES Meaningful interpretation of laboratory data requires an understanding of test results to be expected for patients having various diseases and conditions, as well as for healthy individuals. It would be ideal if such reference ranges for disease never had any areas of overlap with the so-called “reference ranges,” but since that is rarely the case, the next section addresses the interpretation of overlapping result distributions. 1. TAXONOMY OF OVERLAPPING DISTRIBUTIONS Figure 2 shows a pair of curves, representing hypothetical distributions of test results from two distinct populations, one healthy and one diseased with a region of overlap. The horizontal line represents the continuum of possible result values for the analyte we are measuring. The curve on the left represents the distribution of results from the healthy reference population. The curve on the right represents the distribution of results from a group of people known to have the disease. The vertical line represents the upper limit of the reference range. 12 All of the results which fall to the left of the vertical line are called negative. All of the results which fall to the right of the line are called positive. Some diseased patients have “negative” results, since part of their distribution falls to the left of the vertical line. We call this group of results “false negatives.” Conversely, when we defined our reference range we already acknowledged that a small group of healthy individuals would have “false positive” results. To complete the taxonomy, we call the results which accurately reflect the status of the individuals from which they came “true positives” and “true negatives,” respectively. Sensitivity and specificity are performance characteristics of a test. To determine these characteristics, it is necessary to obtain test results on populations in whom the presence or absence of disease has been established by some method independent of this test. Sensitivity is defined as the proportion of diseased subjects correctly classified by the test; i.e., the ability to detect a true positive in a person afflicted with the disease. Sensitivity = TP TP FN Decision Point (e.g. cutoff) Healthy Diseased TN FN TP FP Figure 2: Overlapping Distribution of Test Results for Healthy and Diseased Subjects 13 Specificity is defined as the proportion of healthy subjects correctly classified; i.e., the ability to exclude a diagnosis in a healthy person. Specificity = TN TN FP A convenient format for arranging data is shown in Tables 4 and 5. Note that sensitivity refers only to the diseased population while specificity refers only to the healthy population. The relative sizes of the two populations do not affect sensitivity or specificity. Table 4: Sensitivity and Specificity Number of Subjects with Disease Number of Subjects without Disease TOTALS Number of Subjects with Positive Test Number of Subjects with Negative Test TOTALS TP FN TP + FN FP TN FP + TN TP + FP FN + TN TP + FP + FN + TN Table 5: Example of Sensitivity and Specificity Number of Subjects with Positive Test Number of Subjects with Disease Number of Subjects without Disease TOTALS Number of Subjects with Negative Test TOTALS 68 32 100 sensitivity = 68% 2 98 100 specificity = 98% 70 130 200 Sensitivity and specificity tell us how well a test performs when run on groups of people in whom we already know the diagnosis. In clinical practice, we do not use tests this way. Often, we are running a test on one patient for whom we have not yet made a diagnosis. What we want to know about the test is the odds that the result will correctly classify our patient with respect to the diagnosis we are considering. 2. PREDICTIVE VALUES Predictive values describe the odds that the results of a test will correctly classify an individual with respect to the disease or condition under consideration. To determine predictive values, we need to know the prevalence of the disease in the population we are testing. Prevalence is the fraction of the population which has the disease. 14 The predictive value of a positive test result is the fraction of positive test results which are correct, or the true positives divided by all the positives, both true and false. TP TP FP PV The predictive value of a negative test is the fraction of all negative results which are correct, or the true negatives divided by all the negatives, both true and false. PV TN TN FN Using the hypothetical data provided in Table 5, we can calculate the predictive value of a positive result to be 97% and that of a negative result to be 75%. It is important to recognize the impact of disease prevalence on predictive values. Tables 6 and 7 show two more hypothetical data sets. Both have the same sensitivity and specificity as shown in Table 5, but the prevalence has been decreased, demonstrating the impact on predictive values. In general, as the prevalence of disease increases, the predictive value of a positive test improves. As the prevalence of disease decreases, the predictive value of a negative test improves, and the predictive value of a positive test is diminished by increasing numbers of false positive results. Table 6: Effect of Low Prevalence Diseased Healthy Total Positive 68 20 88 Negative 32 980 1,012 PV+ = 77% PV- = 97% Total 100 1,000 1,100 Sensitivity = 68% Specificity = 98% 100 9% Prevalence 1100 Table 7: Effect of Further Decrease in Prevalence Diseased Healthy Total Positive 68 200 268 PV+ = 25% Negative 32 9,800 9,832 PV- = 99.7% Total 100 10,000 10,100 Sensitivity = 68% Specificity = 98% 100 1% Prevalence 10100 3. INTRAINDIVIDUAL VS. ANALYTICAL VARIATION Up to this point we have focused on the interpretation of individual results relative to group results, or analysis of interindividual variation. We will now focus on determining if therapeutic intervention has changed laboratory values that can be detected analytically. 15 How do you know if my therapy has changed the patient’s lab values? The way to determine if the patient’s lab values have actually changed is to determine if the difference between the first and subsequent measurement is greater than 3 times the standard deviation of the assay. If the difference between the two measurements is greater than 3 times the standard deviation of the assay then you can be 95% confident that the difference between the two measurements is not due to chance (Reference Kaplin LA, Pesce AJ, and Kazmierczak SC, Clinical Chemistry Theory, Analysis, Correlation, 4th edition, St. Louis: Mosby, 2003, page 385). Na mmol/L 114.5 2 SD 114 1 SD 113.5 mean 113 1 SD 112.5 112 11/15 2 SD 11/20 11/25 11/30 12/5 12/10 12/15 12/20 12/25 Date Figure 3: Quality Control Chart for Sodium with a Mean of 113.2 and a Standard Deviation of 0.5 mmol/L G. EXAMPLE Example: A patient had a sodium of 120 mmol/L on day 1. After treatment the patient’s sodium increased to 126 mmol/L. Has the patient become less hyponatremic? In order to answer this question you need to know the analytical variation of the lab’s sodium analysis. This can be determined by calling the lab and asking what the standard deviation of the sodium assay is. The laboratory runs quality control specimens with each batch of samples and will know the analytical variability of the assay in the range of interest. A typical quality control chart is shown in Figure 3. The standard deviation for this sodium control is 0.5 mmol/L. Three times 0.5 mmol/L is 1.5 mmol/L. Since the observed change (6 mmol/L) is greater than 1.5 mmol/L, you can be 95% confident that the patient’s sodium has increased to a degree which can be detected analytically. The primary reason to run controls is to assess whether the test system is functioning properly and generating reliable test results. When the technologists in the laboratory 16 examine the results obtained on the control sample, they are expecting some variation, and they are trying to distinguish between two possible sources of variation: random analytic variation and systematic error. Random analytic variation is inevitable and all its points will fall within a Gaussian distribution. Systematic error occurs when some new variable is introduced, such as deterioration of a reagent, clogging of a tube within the instrument, etc. The problem with systematic error is that it is likely to compromise the accuracy of test results. Each time a technologist sets up an analytical test for patient samples he or she first calibrates the assay with standards containing a known concentration of the analyte. To ensure that the calibration is accurate, the technologist then runs a series of controls. Typically controls are run at three levels, low, normal and elevated concentrations. The technologist then checks the control values to see whether the result falls within the pattern of a Gaussian distribution before analyzing and reporting patient samples. Once the mean and standard deviation have been established for a particular control for a particular analyte, 95% of the control results should fall within ± 2 SD of the mean. About one in 20 results will fall outside these limits, but within 3 SD. If this is just a random event, the next time the control is tested, the result will return to within 2 SD 95% of the time. If it persists outside 2 SD, this is interpreted as most likely a sign of some systematic error, and the technologist proceeds to investigate and take corrective action. Likewise, if any result is more than 3 SD from the mean, it is interpreted as probable systematic error and treated as such. Clinical laboratory technologists do not issue test results if the control results do not fall within established limits. Their objective is to generate the most accurate results possible given the methodology and instrumentation available. The principles of quality control are a major component of their education and training. As Point-of-Care testing becomes more widespread, it is important for pharmacists and other care providers to understand the need to verify the integrity of the test system before using it. 17 18 II LABORATORY DIAGNOSIS OF CARDIAC DISEASE 19 20 A. OBJECTIVES To understand the role of the laboratory in the diagnosis of acute myocardial infarction To understand the time frame in which various protein markers of cardiac damage should be monitored To understand the differences in the various cardiac markers in terms of sensitivity and specificity To understand the role of the laboratory in the diagnosis and monitoring of congestive heart failure B. KEY TERMS Acute myocardial infarction (AMI) - blockage of circulation to heart tissue causing necrosis; frequently associated with pallor, perspiration, nausea, dyspnea, dizziness and ECG changes Acute coronary syndrome (ACS) - a wide range of acute heart conditions including STsegment elevation myocardial infarction, non-ST-segment myocardial infarction, and unstable angina Cardiac markers - proteins used to monitor damage to cardiac tissue, typically cTnI, cTnT, CKMB, and myoglobin Cardiac troponin T (cTnT) - protein released from myocardium due to tissue damage (cardiac specific) Cardiac troponin I (cTnI) - protein released from myocardium due to tissue damage (cardiac specific) Congestive heart failure - cardiac dysfunction syndrome caused by insufficient cardiac output to meet perfusion needs of the patient, characterized by breathlessness, abnormal sodium and water retention, and often edema Creatine kinase (CK) - enzyme released from variety of muscle and other tissues indicating non specific tissue damage Creatine kinase MB (CKMB) - CK enzyme found in highest concentration in heart, also in other tissue Dypsnea - difficult or labored breathing Isoenzyme - a group of different proteins (different sequence) that have the same function Myoglobin - protein released from multiple sources indicating non specific tissue damage Natriuretic peptides - the two main biomarkers used in the diagnosis of heart failure are Btype natriuretic peptide (BNP) and its amino terminal-related fragment NT-proBNP; myocardial stretch causes elevations of these peptides which are diagnostic and prognostic in the setting of heart failure 21 C. BACKGROUND/SIGNIFICANCE It is estimated that about 10 million people present to the emergency department in the US each year with complaints of chest pain. Of these about 6 million people are admitted for workup of AMI and 1.5 million people rule in as having an AMI. Unfortunately about 11,000 of the remaining patients are discharged with an undiagnosed AMI1,2. D. ACUTE MYOCARDIAL INFARCTION Following ischemic injury to cells, cell membrane integrity is damaged allowing intracellular constituents to leak into the blood stream. Important parameters that determine utility of a cellular component as a marker for ischemic cardiac damage include: Intracellular location; cytosolic components generally reach bloodstream faster than structural components Size; smaller components tend to reach bloodstream faster than larger components Tissue specificity; markers that are specific for cardiac tissue provide increased specificity of the assay A variety of proteins are used to detect myocardial damage. The choice of which cardiac markers to analyze, depends on the time elapsed since the suspected beginning of the infarction, which is usually taken as the time of the onset of chest pain or discomfort. Diagnostic problems arise because many of these substances circulate in the blood, albeit at lower levels, in health. Currently the following proteins are monitored when evaluating patients for myocardial infarction: Table 1: Cardiac Markers Marker Cardiac troponin I (cTnI) Cardiac troponin T (cTnT) Creatine kinase MB isoenzyme (CKMB) Myoglobin Reference Range < 0.07 ng/mL * < 0.1 ng/mL * < 10 ng/mL < 170 ng/mL (> 25% increase over 90 min. suggests AMI) * As assays become more sensitive and specific the reference range for troponins continues to decrease. Values greater than 99th percentile of the upper reference limit (greater than 99th percent of the nrmal population) should be considered abnormal.3 1 McCarthy BD, et al., Missed diagnoses of acute myocardial infarction in the emergency department: results from a multicenter study, Ann. Emerg. Med. 22:579-582, 1993 2 Pope JH, et al., Missed diagnoses of acute cardiac ischemia in the emergency department, New Engl. J. Med. 342:1163-1170, 2006 3 Thygesen K, et al, Universal definition of myocardial infarction, Eur Heart J 28:2525-2538, 2007 22 Figure 1: Release of Cardiac Markers following AMI (courtesy of A.H. Wu) Table 2: Protein Elevations in Serum after MI Time After Infarction Serum Protein 1st Elevation (hours) Peak Elevation (hours) CK 4-6 18-36 Myoglobin 2-3 6-9 Troponin I & T 4-6 24-36 Duration of Elevation (days) 3 (~2-3× normal only) 1 depends on extent of damage Definition of acute, evolving or recent MI4 1. Rise and fall of troponin or CKMB with at least one of the following: a. Ischemic symptoms b. Q waves c. ECG changes (increase or decrease of ST segment) d. Coronary artery intervention (e.g., coronary angioplasty) 2. Pathological findings of AMI 1. TROPONIN The diagnosis of AMI relies heavily on the analysis of troponins. Of the markers currently available, cTnI and cTnT offer the highest degree of cardiac specificity. 4 Alpert JS, et al., Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction, J. Am. Coll. Cardiol. 36:959-969, 2000 23 Troponin is a regulatory protein complex located on the thin filament of the contractile apparatus and consists of 3 protein subunits: troponin T, troponin I and troponin C which act to regulate muscle contraction. Troponin T binds to tropomyosin, Troponin I inhibits myosin ATPase, and Troponin C binds to calcium. Unlike other cardiac markers that are used to detect cardiac damage, cTnI and cTnT have different isoenzymes from those found in skeletal muscle, and thus they are specific for cardiac injury. In addition to detecting an AMI, elevations of either cTnT or cTnI have prognostic value. Marginally elevated concentrations of cTnT and/or cTnI are associated with future adverse cardiac events. It is important to realize that currently there are many different cTnI assays and reference ranges vary widely depending on who manufactures the assay. At the VA hospital cTnI values between 0.07 and 0.20 ng/mL suggest myocardial ischemia or early infarction serial testing is indicated. Values greater than 0.2 ng/mL are consistent with MI, indicating the patient should undergo serial testing. Reference range: < 0.07 ng/mL. Since there is only one assay available for cTnT, the reference ranges have less variability between laboratories. Serum cardiac troponin T > 0.1 ng/mL suggests myocardial damage, an MI, or high risk for future myocardial events. Many end stage renal failure patients on hemodialysis (60%) have elevated cTnT and/or CTnI, which does not represent an AMI, but is associated with future adverse cardiac events. The percentage of end stage renal failure patients on dialysis that are cTnI positive is less than that observed with cTnT. Figure 2: Structure of Troponin Complex Elevations of cardiac troponins are diagnostic of heart tissue injury but do not always indicate myocardial infarction. In the setting of ischemic symptoms a rise and fall indicated myocardial infarction, but elevations can be caused by other factors such as heart failure, drug toxicity, myocarditis, etc.). 24 2. CREATINE KINASE (CK) ISOENZYMES IN MYOCARDIAL INFARCTION CK (molecular weight 86 kilodaltons (KD)) is an enzyme responsible for the conversion of creatine into phosphocreatine, the energy source for muscle contraction. Since CK is found in all muscle tissue, elevations of the total activity of this enzyme are not specific for cardiac damage. However, CK is a dimer and there are three different forms of the enzyme (isoenzymes) with varied tissue distribution. In skeletal muscle the predominate form of CK is CKMM. In heart muscle CKMM is also the predominate form, but CKMB makes up about 20% of the CK activity, whereas in skeletal muscle CKMB is generally about 1% of the CK activity. The third form of CK, which is not used as a cardiac marker, is CKBB. Phosphocreatine Creatine Spontaneous cyclization • high energy compound for muscle contraction Creatine Kinase (CK) • monitored to diagnose muscle damage • MB isoenzyme more specific for myocardium Creatinine • used to monitor renal function • 1-2% of muscle mass per day Figure 3: Relationship between Creatine, Creatine Kinase, Phosphocreatine, and Creatinine Since cardiac tissue has the highest percentage of CKMB isoenzyme (22% CKMB and 77% CKMM), this isoenzyme is monitored in the diagnosis of AMI. The key finding in myocardial infarction is an elevation of CKMB to greater than 10 ng/mL, approximately 6-48 h after the onset of chest pain. It is important to remember that CKMB is not cardiac specific, as about 1% of the CK found in skeletal muscle tissue is CKMB. The tissue distribution of CK is shown on the figure on the next page. The lack of specificity of CKMB for cardiac tissue has led the use of the CK index (“relative index). CK Index = CKMB mass (ng/mL) × 100 Total CK activitiy (U/L) CKMB within reference range and an index < 2.5 is considered normal CKMB above reference range and an index > 2.5 suggests cardiac damage CKMB above reference range and an index < 2.5 suggests skeletal muscle damage 25 Other reasons for elevations of CKMB are: Myocarditis Polymyositis Muscle trauma Muscular dystrophies Malignant hyperthermia Shock Cardiac surgery Surgery Severe angina Idiopathic (rare) Coronary insufficiency Other myopathy Dermatomyositis Drug-induced rhabdomyolysis In these cases the use of the CK index helps distinguish between cardiac and non-cardiac causes of elevated CKMB. It is advisable that during the first 24 hours at least three samples be drawn for in order to pinpoint the peak of the elevation of CKMB. Demonstration of the peak (rise and fall) within 48 h increases the specificity of the test since it occurs in MI but not in muscle disease in which values plateau. An elevation of CKMB above 10 ng/mL with an elevated CK index indicates myocardial infarction with very high specificity. Figure 4: Relative Activities of the CK Isoenzymes in Selected Human Tissues (adapted from Tsung, 1976) 3. MYOGLOBIN Myoglobin is a low-molecular weight protein (about 18 KD) found in all skeletal and cardiac muscle and is involved in oxygen binding. Due to its small size, it appears in serum rapidly after release from injured muscle and is cleared into the urine. Since myoglobin is found in most tissues, it has the least specificity of any of the cardiac 26 markers. False positive results for diagnosis of myocardial infarction may be encountered with skeletal muscle injury and renal failure. Serum myoglobin > 70 ng/mL is an early, but not specific indicator of myocardial infarction and thus should never be used by itself. For patients who arrive within several hours of onset of chest pain, myoglobin is analyzed on admission and 90 minutes later. If the concentration has increased by at least 25%, this is consistent with an early MI. This finding should be confirmed with more specific techniques (cTnI, cTnT, or CKMB). Table 3: Table of Sensitivity and Specificity of Various Markers of Cardiac Injury (from Wu, A.H., Diagnostic Enzymology in Clinical Laboratory Medicine, 1994) Sensitivity (%) Marker 2 - 8 h 8 - 24 h 24 -72 h Myoglobin 95 75 0 CKMB 60 95 98 Troponin 75 95 98 72 h 0 50 98 Specificity (%) 70 95 > 95 Drug Therapy for AMI Thrombolytics - Streptokinase, Anistreplase, Alteplase, Reteplase Compounds with antiplatlet activity and Anticoagulants - aspirin, heparin, glycoprotein IIb/IIIa inhibitors -blockers - propranolol Vasodilators - nitrates Analgesics - morphine or meperidine if not relieved by nitroglycerin Other therapies - oxygen, antiarrthymics, stool softeners, percutaneous transluminal angioplasty E. CONGESTIVE HEART FAILURE (CHF) An estimated 4.8 million Americans have CHF with an additional 550,000 new cases each year. Of the CHF patients, about 300,000 patients will die each year 5. Symptoms of CHF are often nonspecific (dyspnea) and consequently this syndrome is sometimes difficult to diagnose. Analysis of B-type natriuretic peptides (BNP) have been approved as diagnostic markers for CHF. BNP is synthesized as a pro-molecule consisting of 108 amino acids, proBNP (1-108). With left ventricular dysfunction (volume overload and myocardial stretch) pro-BNP (1-108) is released from the left ventricle. During this process pro-BNP (1-108) is cleaved into the active hormone BNP (77-108) and an inactive N-terminal fragment known as NT-pro-BNP (1-76). Assays with antibodies that recognize to the carboxy-terminal of the molecule, BNP (77-108), or the amino-terminal, NT-pro-BNP are used to diagnose and monitor treatment of CHF. Both NT-pro-BNP and BNP concentrations have been shown to correlate with the degree of cardiac failure, but these assays have different reference ranges. Thus it is important to know which type of analysis is being performed on your patients. 5 American Heart Association, 2009 Heart and Stroke Statistical Update, Dallas, Texas: American Heart Association, 2001 [available at <http://www.americanheart.org/presenter.jhtml?identifier=1928>] 27 Table 4 Marker Reference Range BNP (77-108) < 100 pg/mL NT-pro-BNP (1-76) if less than 75 years old < 125 pg/mL NT-pro-BNP (1-76) if greater than 75 years old < 450 pg/mL 28 F. CASE STUDIES CASE 1 (Can. J. Emerg. Med. 8:289-294, 2006) A 29-year-old woman was prescribed penicillin V for pharyngitis by her primary care provider 2 days before ED presentation. On that same day she took 1 dose in the afternoon and a 2nd dose between 6 and 8 p.m. that night. She presented to the ED at approximately 1:45 am complaining of lip swelling and shortness of breath. Lip swelling commenced approximately 10 hours after the initial dose. She denied chest pain or pressure, dyspnea on exertion, and cough or sputum production. Physical exam revealed an anxious young woman in moderate respiratory distress. Initial vital signs were: temperature 36.7°C, blood pressure 113/64 mmHg, pulse 73 beats/min, respiratory rate 28, O2 sat 94% on room air. Immediate medical treatment included diphenhydramine 50 mg IV, methylprednisolone 125 mg IV, and cimetidine 300 mg IV. As she continued to be tachypneic and distressed, she was given 1:10 000 epinephrine, 0.1 mg (1 cc) IV. The dose was confirmed, as it was a preloaded 10-cc syringe. She began to complain of severe pressure-type central chest pain. Results of an ECG revealed ST elevations in several leads. Laboratory results (Reference Ranges): Time Troponin I CK CKMB (< 0.04 ng/mL) (20-212 IU/L) (0-4.5 ng/mL) 3:45 a.m. < 0.04 66 0.1 8:40 a.m. 1.99 85 3.3 3:32 p.m. 1.63 106 3.3 1. Calculate the relative index for the three time points. 2. Based on these laboratory findings did the patient rule in for an MI? 3. How do you explain the normal values of CK and CKMB? 4. Is this a common ADR for epinephrine? 29 CASE 2 A 57-year-old man presented to the emergency department with a 1-hour history of shortness of breath, coughing, and “chest tightness.” He states that his symptoms appeared suddenly while he was watching television on his couch. He describes a constant “squeezing” sensation in his chest limited to the parasternal region associated with a paroxysmal nonproductive cough. He describes his shortness of breath as “difficulty getting air,” denying any pain on inspiration or expiration. He denies any similar episode in the past. His past medical history is significant for diabetes mellitus, hypertension, and severe peripheral vascular disease. In the past 6 years his right leg was amputated just above the knee and the left leg below the knee. He denies any syncopal episode, thigh, or abdominal swelling. He sleeps on one pillow at night and usually does not wake up to urinate. He used to drink alcohol heavily but has not had a drink since 1978. He denies any tobacco or illicit drug use. Vital Signs: T 97 F, P 125 BPM, RR 28/min, BP 179/102 mmHg Physical Exam: Neck: No jugular venous distension (JVD) Heart: Sinus tachycardia, regular rhythm; grade I/VI nonradiating systolic ejection murmur at the left sternal border Lungs: Fine rales in the lower ½ of each lung field; no rhonchi or friction rubs Chest wall: No tenderness to palpation Abdomen: No ascites or hepatosplenomegaly; no tenderness CXR: Bilateral fluffy infiltrates consistent with pulmonary edema EKG: Sinus tachycardia; no acute changes from previous EKG; no S-T segment depression or elevation (on admission) Laboratory Tests: CKMB (ng/mL) Troponin I (ng/mL) Myoglobin (ng/mL) Admission 30 min 60 min 90 min 2.7 4.3 5.3 6.5 9.8 11.4 8.8 < 0.5 < 0.5 < 0.5 0.8 3.6 8.2 5.9 49 98 165 184 3 hrs 129 8 hrs 86 1. How do you interpret these data? Are they diagnostic? 2. Given the above results, graph their relative crescendos, peaks, and durations of elevation. 3. What do the lab tests tell you about the chronology of events? 30 12 hrs 56 III LABORATORY DIAGNOSIS OF DYSLIPIDEMIA 31 32 A. OBJECTIVES To understand the current National Cholesterol Education Program guidelines To understand risk related target goals To be able to describe the various sources of lipids To understand how the laboratory is used to monitor drug therapy B. KEY TERMS Chylomicron - class of lipoprotein that transports exogenous (dietary) triglycerides and cholesterol for metabolism Dyslipidemia - disorder of lipoprotein metabolism HDL - high density lipoprotein IDL - intermediate density lipoprotein LDL - low density lipoprotein Lipid - heterogeneous group of fat and fat like substances, poorly soluble in aqueous solutions Lipoprotein - lipid protein complex for transporting lipids in the blood NCEP - National Cholesterol Education Program Triglycerides - three fatty acids esterified to a glycerol backbone VLDL - very low density lipoprotein C. BACKGROUND SIGNIFICANCE Lipids and lipoproteins are essential for a variety of biochemical processes and serve as hormones and energy reserves in addition to forming key elements of cell membranes. In disease states, lipids are most frequently monitored in association with atherosclerosis. Laboratory testing is essential for determining when to institute drug therapy and for monitoring disease progression. Understanding the role of the laboratory in management of dyslipidemia is essential for pharmacists as atherosclerosis and cardiac disease are the leading cause of death in developed countries. 33 Figure 1: Exogenous Lipoprotein Metabolism (TG, triglyceride; CE, cholesterol ester; FC, free cholesterol; PL, phospholipids; FA, fatty acids; LPL, lipoprotein lipase; B, apolipoprotein B-48; A, apoliprotein A-I; C, apoliprotein C-II; E, apoliprotein E; from Arch. Pathol. Lab. Med. 110:694-701, 1986) Cholesterol and fatty acids are absorbed from the GI tract as chylomicrons (Figure 1). Chylomicrons are primarily triglycerides (90%) combined with cholesterol and apolipoproteins B-48 and A. In combination with HDL, apolipoprotein C, apolipoprotein E and lipoprotein lipase, a small percentage of the free fatty acids are released from the chylomicron triglyceride component. The free fatty acids are then taken up by either muscle or adipose cells. The remaining chylomicron remnant which contains 80-90% of the initial triglyceride component can then be internalized by hepatic cells. Hepatic cells synthesize triglyceride rich VLDL which is then released into circulation (Figure 2). Apolipoprotein CII activates lipoprotein lipase which releases free fatty acids to endothelial cells. The remaining VLDL remnant can either be taken back up by hepatocytes or be converted into IDL. Further metabolism results in LDL, where most of the triglyceride component of VLDL has been replaced with cholesterol. The major components of the various lipoprotein classes are shown in Table 1. Table 1: Major Lipids and Protein Components of Lipoprotein Classes Variable Chylomicron VLDL IDL LDL HDL Major lipids TG TG TG, CE CE Phospholipids Major proteins AI, B-48, CI-CIII B-100, CI-CIII, E B-100, E B-100 AI, AII From a clinical perspective, monitoring and reducing LDL is a primary goal of therapy. 34 Figure 2: Endogenous Lipoprotein Metabolism Pathway (IDL, intermediate-density lipoprotein; LCAT, lecithin choles- terol acyltransferase; B, apoliprotein B-100; E, apoliprotein E; from Arch. Pathol. Lab. Med. 110:694-701, 1986) Table 2: Adult Classifications of LDL, Total and HDL Cholesterol (mg/dL) LDL Cholesterol < 100 Optimal 100-129 Near optimal 130-159 Borderline 160-189 High Very high 190 Total Cholesterol < 200 Desirable 200-239 Borderline high High 240 HDL Cholesterol < 40 Low > 60 High D. HYPERLIPIDEMIA 1. FROM THE NCEP GUIDELINES6 Risk assessment-measure LDL on anyone over 20 once every 5 years Clinical judgment applied to individuals should always take precedence over general management principles In a meta-analysis of dietary trials, dietary lowering of serum cholesterol produces as much CHD risk reduction as did drugs, commensurate with their respective degree of cholesterol lowering 6 Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), Executive Summary, Bethesda, MD: National Heart Lung and Blood Institute, National Institutes of Health Publication No. 01-3670, 2001, <http:// www.nhlbi.nih.gov/guidelines/cholesterol/index.htm> 35 Since safety does not appear to be an issue for short term risk reduction in primary prevention with LDL lowering drugs, the determining factor for the lower risk cut point for drug recommendation will be cost effectiveness 2. LABORATORY ASSESSMENT OF HYPERLIPIDEMIA Total cholesterol- can be measured on fasting or non-fasting specimens Total cholesterol is determined from the amount of cholesterol found in HDL, LDL and VLDL HDL - can be measured on fasting or non-fasting specimens Triglycerides- fasting required LDL - fasting required LDL - if triglycerides < 400 then LDL is calculated using Friewald formula: Calculated LDL = chol - HDL - TG/5 If triglycerides > 400 then LDL is measured directly Fasting = 9-12 hours of not eating or drinking (except water) Blood should be drawn with the patient sitting (5 minutes) in order to avoid hemoconcentration 3. INTERPRETATION OF LIPID PANELS Baseline lipid should be the average of two measurements, 1-4 weeks apart, before instituting drug therapy Baseline measurements should also include liver enzymes (ALT or AST), CK and medical history Initial follow-up should occur 6-8 weeks after drug therapy when response should be maximal 4. RISK FACTORS THAT MODIFY LDL GOALS Cigarette smoking Hypertension (BP > 140/90 mmHg or on antihypertensive medicine) Low HDL cholesterol* (< 40 mg/dL) Family history of heart disease Age (men 45, women 55) (* HDL > 60 is a negative risk factor, it removes one risk factor from the total count) Table 3: Risk Categories and LDL Goals Risk Category LDL Goal (mg/dL) Very high risk < 70 CHD (or equivalents) < 100 Multiple (≥ 2) risk factors < 130 Zero to one risk factor < 160 The LDL goal is determined by risk factors. The very high risk category includes patients with a recent MI or a combination of cardiovascular disease and diabetes. Patients with 36 established CHD (coronary heart disease) or equivalents (e.g., diabetes) have an LDL goal of < 100 mg/dL, whereas for people with fewer risk factors higher levels of LDL are considered acceptable. 5. SECONDARY CAUSES OF HYPERLIPIDEMIA Diabetes Hypothyroidism Obstructive liver disease Chronic renal failure Drugs (progestins, anabolic steroids, corticosteroids) 6. SECONDARY PREVENTION OF CHD (CONFIRMED CHD PATIENTS) In patients with confirmed CHD, target LDL is < 100 mg/dL Good evidence that lowering lipids is beneficial for preventing additional adverse cardiac events (myocardial infarction, death) Patients with established CHD (or equivalents) should have drug therapy if LDL > 130 mg/dL as lifestyle changes unlikely to reduce LDL to < 100 mg/dL In these patients, LDL lowering reduces risk of strokes AMI patients should have lipids on admission or within 24 hours of presenting; if LDL is > 130, then discharge on TLC and drug therapy 7. THERAPEUTIC LIFESTYLE CHANGES (TLC) Reduced intakes of saturated fats and cholesterol Therapeutic dietary options to enhance LDL lowering (plant stanols/sterols and increased viscous fiber) Weight control Increased physical activity 8. METABOLIC SYNDROME Higher risk for diabetes and CHD Obesity Elevated triglycerides Decreased HDL Elevated blood pressure (> 130/85) Elevated fasting plasma glucose 9. TREATMENT TLC for approximately 3 months; recheck labs, if not at goal then consider drug therapy Drug therapy: start statin or bile acid sequestrant or nicotinic acid 37 Table 4: Reference Ranges and Lab Testing Frequency Risk Level CHD or risk equivalent 2+ risk factors 0-1 risk factor 0-1 risk factor LDL Goal Observed LDL (mg/dL) (mg/dL) Repeat Lipid Labs < 100 < 100 < 1 year < 130 < 130 2 years < 160 130-159 2 years < 160 < 130 5 years 10. THE MAJOR CLASSES OF DRUGS FOR CONSIDERATION ARE: A) HMG CoA reductase inhibitors (statins) - lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin 1) LDL (18-55%), Reduce TG (7-30%) HDL (5-15%) 2) Contraindications: liver disease 3) Adverse effects: myopathy (check creatine kinase initially), increased transaminases (rarely > 3 times upper limit of normal) B) Bile acid sequestrants - cholestyramine, colestipol, colesevelam 1) LDL (5-30%), No effect or TG (7-30%) HDL (3-5%) 2) Contraindications: elevated TG (absolute > 400 mg/dL, relative > 200 mg/dL) 3) Adverse effects: GI distress, absorption of other drugs C) Nicotinic acid (alters lipoprotein synthesis) - crystalline, timed-release preparations, Niaspan® 1) LDL (5-25%), TG (20-50%) HDL (15-35%) 2) Contraindications: severe liver disease, severe gout 3) Adverse effects: (often limit prolonged use): flushing, hyperglycemia, gout, hepatotoxicity D) Fibric acid derivatives (peroxisome proliferator-activated receptor-alpha, PPAR-alpha agonist) - gemfibrozil, fenofibrate, clofibrate 1) LDL (5-20%) in patients w/o hypertriglyceridemia, TG (20-50%), HDL (10-35%) 2) Contraindications: severe hepatic or renal insufficiency 3) Adverse effects: dyspepsia, GI distress, cholesterol gallstones, myopathy E) Cholesterol absorption inhibitors - ezetimibe (Zetia®) 1) LDL (17%), no change in TG or HDL 2) Contraindications: statin contraindications apply when used in combination with a statin 3) Adverse effects: diarrhea, upper respiratory tract infection, arthralgia 38 E. CASE STUDIES CASE 1 (Ann. Pharmacother. 37(7):1032-1035, 2003) An 83-year-old white man with a diagnosis of acute myeloid leukemia (AML) received remission induction chemotherapy consisting of anti-CD33 antibody conjugated with a cytotoxic anti-tumor antibiotic, calicheamicin (gemtuzumab ozogamicin). His past medical history included congestive heart failure, hyperlipidemia, and hypothyroidism. He had been taking simvastatin for nearly 2 years. The patient tolerated the chemotherapy well and was discharged on his previous medications, including simvastatin 40 mg once daily, digoxin 0.25 mg once daily, and levothyroxine 0.075 mg once daily, as well as levofloxacin 500 mg once daily, acyclovir 400 mg twice daily, and fluconazole 400 mg once daily, given as prophylactic antimicrobial therapy in the setting of neutropenia. One week after discharge, the patient presented with severe generalized muscle weakness. There was no history of trauma, fever, alcohol use, seizures, or systemic inflammatory diseases. He was afebrile, and other vital signs were also normal. Examination revealed 2 of 5 strength in all 4 extremities and decreased ankle reflexes bilaterally. Notable laboratory values included serum creatine kinase (CK) 52,716 U/L, CK MB fraction 81.5 ng/mL, serum creatinine 1.2 mg/dL (baseline 1 wk earlier 0.9 mg/dL), blood urea nitrogen 30 mg/dL (baseline 1 wk earlier 16 mg/dL), troponin I < 0.4 µg/L, calcium 8.3 mg/dL, potassium 5.1 mEq/L, alanine aminotransferase 724 U/L, aspartate aminotransferase 2367 U/L, alkaline phosphatase 187 U/L, thyroid-stimulating hormone (TSH) 37.7 µU/mL, and unbound thyroxine 0.61 ng/dL. Urinalysis showed brown urine, pH 5.0, abundant pigment casts, and a red supernatant after centrifugation. 1. Based on the CKMB being greatly elevated (normal is < 12 mg/dL) did this patient suffer a myocardial infarction? Is the elevated CKMB consistent with the troponin result? 2. How do you interpret the creatine kinase concentrations in this patient? If the patient is treated effectively how long will it take to normalize the CK values? 3. Why is the serum potassium slightly elevated? 4. How do you interpret the transaminase (ALT and AST) values for this patient? 5. How do you interpret the thyroid function tests? 6. How do you interpret the urinalysis data? 7. Is this a common finding with these types of drugs? 8. How should this patient be treated? 39 CASE 2 (CMAJ 167(11):1261-1266, 2002) A 41-year-old man presents for assessment of severe hypertriglyceridemia. He has previously been well. His lipid levels were checked because his serum appeared milky on screening for dyslipidemia. He has no history of abdominal pains, his medical history is unremarkable (in particular, no diabetes mellitus, hypothyroidism, obesity or pancreatitis), and he has no family history of premature coronary artery disease. He does not drink alcohol excessively and is a nonsmoker. The physician records his height (180 cm), weight (80 kg) and abdominal circumference (86 cm). Other findings on physical examination are unremarkable. The fasting lipid profile reveals a total cholesterol level of 300 (normally < 200) mg/dL, a high-density lipoprotein (HDL) cholesterol level of 9 (normally > 35) mg/dL and a triglyceride level of 2672 (normally < 200) mg/dL. The patient had previously received treatment with 2 statins, in increasing doses, without a decrease in triglycerides. 1. How should the patient’s lipid profile be classified? 2. What other investigations should be performed? 3. How should the patient’s dyslipidemia be managed? 40 IV LABORATORY DIAGNOSIS OF RENAL DISEASE 41 42 A. OBJECTIVES To understand the use of creatinine and urea nitrogen in diagnosis and monitoring of renal function To be able to differentiate renal tubular dysfunction from glomerular dysfunction To be able to calculate creatinine clearance B. KEY TERMS Albuminuria - the presence of albumin in urine Anuria - lack of urine output (< 50 mL per day) Azotemia - an excess of urea, creatinine and other nitrogenous end products of amino acid metabolism in blood Bowman’s capsule - a structure consisting of glomeruli and extended opening of proximal tubule Casts - protein aggregates, outlined in the shape of renal tubules, secreted into the urine Creatinine - a spontaneous breakdown product of muscle creatine, used to monitor renal function Creatinine clearance - an estimate of the glomerular filtration rate obtained by measurement of the amount of creatinine in the plasma and its rate of excretion into the urine End-stage renal disease (ESRD) - a condition in which renal function is inadequate to support life Glomerular filtration rate (GFR) - the rate in milliliters per minute that substances such as creatinine and urea are filtered through the kidney’s glomeruli (mL/min); a reflection of the number of functioning nephrons; estimated by the creatinine clearance (see above) Glomerulonephritis - nephritis accompanied by inflammation of the capillary loops in the glomeruli of the kidney; occurs in acute, subacute, and chronic forms and may be secondary to hemolytic streptococcal infection; evidence suggesting possible immune or autoimmune mechanisms Glomerulus - a tuft of blood vessels found in the nephron of the kidney that are involved in the filtration of the blood Hematuria - blood in the urine Hemodialysis - the exogenous removal of certain elements from the blood by virtue of the difference in the rates of their diffusion through a semipermeable membrane (for example, by means of a hemodialysis filter) 43 Isosthenuria - urine with a fixed specific gravity in range of 1.008 to 1.012 Microalbuminuria - low-grade, dipstick-negative increase in urine albumin excretion, useful in monitoring renal status of individuals prone to renal impairment from diseases such as diabetes Nephritis - inflammation of the kidney with focal or diffuse proliferation or destructive processes that may involve the glomerulus, tubule, or interstitial renal tissue Nephron - the anatomical and functional unit of the kidney, consisting of the renal corpuscle, proximal convoluted tubule, descending and ascending limbs of Henle’s loop, distal convoluted tubule, and collecting tubule Nephrotic syndrome - general name for a group of diseases involving increased glomerular permeability, characterized by massive proteinuria and lipiduria with varying degrees of edema, hypoalbuminemia, and hyperlipidemia Oliguria - decreased urine output (< 400 mL per day) Peritoneal dialysis - hemodialysis through the peritoneum, the dialyzing solution being introduced into and removed from the peritoneal cavity as either a continuous or an intermittent procedure Pyelonephritis - inflammation of the kidney and its pelvis Pyuria - the presence of pus (an inflammation fluid with leukocytes and dead cells) in the urine Renal threshold - the plasma concentration of a substance above which it will be excreted into the urine Tamm-Horsfall protein - a mucoprotein produced by the ascending limb of the loop of Henle that is a normal constituent of urine and is the major protein constituent of urinary casts Urea - major nitrogen containing product of protein metabolism, used to monitor renal function Uremia - an excess in the blood of urea, creatinine, and other nitrogenous end products of protein and amino acid metabolism; more correctly referred to as azotemia C. BACKGROUND/SIGNIFICANCE Most renal disease causes no symptoms until 50-75% of kidney function is destroyed. Lab tests often provide the initial recognition of renal impairment and can be used to direct further therapy. In therapeutics, knowledge of renal function is essential for recommending proper doses of prescription drugs. Many drugs are renally cleared and decreased renal clearance can lead to toxicity. Dose adjustments are based on assessments of renal function. 44 D. EXCRETORY FUNCTION OF KIDNEY The kidney serves to rid the body of undesirable end products of metabolism. The nonprotein nitrogenous compounds that are excreted by the kidney, are listed below: Contributors Urea Uric acid Creatinine Creatine Amino acids Ammonia Other Minor Contributors Nucleotides Purine Polypeptides Glutathione The two most popular screening tests for renal function are serum urea nitrogen and serum creatinine. Better measures of glomerular function are clearance tests. This is especially true in the aged, where serum creatinine measurements are less reliable due to decreasing muscle mass. In uremia (kidney failure) the increase is mainly in urea, creatinine and uric acid. Uric acid and the other nonprotein nitrogenous compounds are measured rarely in this context. Serum urea nitrogen (SUN) is normally ~45% of total non-protein nitrogenous compounds, but may be 80% in some disease states. In hepatic failure the ratio of non-urea/urea nitrogen increases, because of the inability of the liver to synthesize urea and to deaminate amino acids. 1. UREA NITROGEN Major nitrogen-containing metabolic product of protein catabolism Primarily synthesized by hepatocytes Freely filtered by glomeruli, reabsorbed (amount varies) by tubules Historically, urea was reported as blood urea nitrogen (BUN) and this terminology has been incorrectly carried over to the present. Urea nitrogen is now measured using serum and is reported as “serum urea nitrogen” (SUN). Urea is synthesized mostly in the liver as a by-product of the deamination of amino acids. Urea is filtered by the glomeruli; however, about 40-70% (amount depends on urine flow) is reabsorbed by passive diffusion into blood across the renal tubular epithelium. For this reason, urea clearance tests are less informative than creatinine clearance tests and have been discontinued. 45 Reference Range for serum urea nitrogen: 8-23 mg/dL The serum urea nitrogen level is greatly influenced by diet. Table 1 Daily Protein Intake Urea-N Average (mg/dL) (g/kg body weight) Low protein diet: 0.5 ~5 Average protein diet: 1 ~12 High protein diet: 2 ~22 SUN is not a sensitive indicator of renal dysfunction because renal function must be reduced by more than 50% to result in a rise of SUN. 2. CREATININE Derived from muscle creatine (about 1-2% of total muscle mass per day) Amount excreted daily is fairly constant and independent of urinary volume Average men excrete 1.5 g/d into the urine; women less; athletes more Patients with hepatic disease, muscular dystrophy, paraplegia and poliomyelitis may excrete less creatinine due to decreased production. It should be noted that many laboratories use the alkaline picrate (Jaffe) method for measuring creatinine. Reference Range for serum creatinine: M: 0.6 - 1.3 mg/dL F: 0.5 - 1.1 mg/dL 3. GLOMERULAR FILTRATION RATE A useful assessment of renal function is the measurement of the glomerular filtration rate (GFR), especially in older people. The inulin clearance rate closely approximates the true GFR since inulin is freely filtered by the glomerulus with minimal tubular absorption or secretion. However, this test is difficult to perform routinely. Measuring the clearance of endogenous creatinine (clearance of creatinine produced by metabolic processes) is much more practical and convenient but less accurate. Creatinine, for the most part, is freely filtered. Normally, only small amounts (< 6%) are reabsorbed by the tubules and an equal amount may be secreted bythe tubules. The endogenous creatinine clearance rate is computed by the following formula: Ccreat = Ucreat Screat × V × 1.73 m2 A where Ccreat = creatinine clearance rate in mL/min, Ucreat = urinary creatinine in mg/dL, V = urine flow in mL/min (volume of timed urine/collection time), and S creat = serum creatinine in mg/dL. Since creatinine clearance is related to patient size, a more accurate 46 formula includes an estimation of body surface area in square meters obtained from a nomogram using the height and weight of the patient. A = body surface area in m2, and 1.73 m2 is the average body surface area. In essence this formula “normalizes” the clearance to that of a normal-sized person. Patients being tested for the GFR must be well hydrated to provide a urine flow of > 2 mL/ min. Reference Range: male = 117 20 mL/min; female = 95 20 mL/min Serum creatinine (mg/dL) The relationship between serum creatinine and creatinine clearance is logarithmic (see Figure 1). Thus, initially, for small numeric changes in serum creatinine, there are significant numeric changes for creatinine clearance. In later stages of uremia, small numeric changes in the clearance are associated with significant changes in serum creatinine. Note the decrease in the number of nephrons with decrease of clearance and increase in serum creatinine. 8 4 2 1 120 15 30 60 Creatinine Clearance (ml/min) 0.5 1.0 2.0 Remaining Nephrons (millions) Figure 1: Relationship between serum creatinine, creatinine clearance and number of remaining nephrons. The endogenous creatinine clearance rate is primarily an estimate of GFR; however, a small amount of tubular secretion augments glomerular filtration. Therefore, total urinary 47 creatinine is slightly higher than the amount actually filtered by the glomeruli. The amount of urinary creatinine derived from tubular secretion rises proportionally in renal failure with an increase in serum creatinine. With advancing renal failure and increase in serum creatinine, the tubular secretion proportionally increases up to 40-60%. From an interpretation standpoint this is important. With normal or early renal failure creatinine clearance approximates true GFR. With advanced renal failure creatinine clearance overestimates true GFR due to the increased amount of creatinine in urine due to tubular secretion caused by the high concentration of creatinine in circulation. Alternatively, more accurate isotopic methods for measuring GFR can be utilized, e.g. the clearance of injected 51Cr-labelled EDTA from the blood. This compound is completely filtered by the glomeruli and is not secreted or reabsorbed by the tubules. However, this method requires a special laboratory equipped to handle and measure radioactive isotopes. An alternative to measuring GFR is to use the Cockroft and Gault equation7 to estimate creatinine clearance based on a single measurement of serum creatinine. Where S creat is serum creatinine (mg/dL) and weight is in kilograms. Ccreat (males) = (140-age)(Weight) (Screat) (72) Ccreat (females) = (140-age)(Weight) (0.85) (Screat) (72) E. TUBULAR FUNCTION The most often used function tests are renal concentrating power and the ability to produce an acid urine (in suspected renal tubular acidosis). URINE OSMOLALITY AND RENAL CONCENTRATING ABILITY Osmolality refers to the concentration of osmotically active particles (osmolutes) in solution, expressed in mOsm/kg of water. Urine osmolality varies widely in health, between about 60 and 1250 mOsm/kg. The failing kidney loses its capacity to concentrate urine. A patient with polyuria due to chronic renal failure (CRF) is unable to produce either a dilute or a concentrated urine. Instead, urine osmolality in these patients is generally within 50 mOsm/kg of the plasma osmolality (i.e., between about 240 and 350 mOsm/kg). Urine osmolality is a measure of concentrating power of the kidney. Urine specific gravity is usually directly proportional to osmolality. Both give misleadingly high results if there is significant glycosuria or proteinuria. The error can be mathematically corrected. Measurement of osmolality with an osmometer is more accurate, but also more difficult than specific gravity measurements. Random testing of either specific gravity or osmolality is not very informative, due to the effect of fluid intake. Repeated measurements, or measurements under controlled fluid intake are more reliable. Specific gravity values of more than 1.025 or osmolality values more than 875 mOsm/kg indicate adequate renal concentrating ability. Recurring values of 1.010 (1.008 - 1.012) indicate isosthenuria (fixed specific gravity). This finding 7 Tietz Textbook of Clinical Chemistry, 3rd edition, 1999, p 1242 48 suggests loss of tubular concentrating and diluting ability and is frequently a prelude to anuria. F. SUN/CREATININE RATIOS IN VARIOUS CONDITIONS Normal ratio: ~12-20 (or 10-18 with less specific methods) In practice, the greatest increase in the ratio may be seen in prerenal azotemia, where ratios of 30/1 or 35/1 may be observed. However, other conditions may also change the ratio, depending on the rate of urea synthesis, kidney blood flow, or glomerular filtration rate. When evaluating SUN/creatinine ratios realize that SUN production is dependent on available protein (increased protein intake increases the ratio) and liver function (decreased liver function lowers the ratio). In addition, the ratio is affected by the specificity of the creatinine method. (Less specific methods give higher creatinine values.) The ratio is increased in conditions in which there is increased urea synthesis, as observed in the presence of blood in the GI tract, in muscle wasting disease, and in severe tissue trauma. Other conditions such as intraperitoneal extravasation of urine and urinary enteric fistulas lead to greater urea reabsorption. Increased tubular reabsorption of urea occurs with decreased tubular flow as a result of dehydration, decreased cardiac output, or shock (= prerenal azotemia), or due to renal disease, such as early acute glomerulonephritis, malignant nephrosclerosis, or postrenal obstruction. Decreased ratios are seen in the presence of decreased urea synthesis (chronic glomerulonephritis with protein deficiency, severe hepatic insufficiency, and starvation) and decreased urea reabsorption (overhydration and rapid hydration). The decrease in ratio due to hemodialysis is caused by the more efficient dialysis of urea vs. creatinine. In acute tubular necrosis, urea and creatinine are equally and passively returned to the tubular blood. Therefore, the ratio is decreased. G. RENAL FAILURE Acute renal failure occurs rapidly and is potentially reversible if the initial illness or insult is survived (60% survival rate). Chronic renal failure usually develops over years in an insidious manner leading to endstage renal disease requiring lifelong dialysis or renal transplant. Azotemia (increase of urea or other non-protein nitrogenous - compounds) is divided into 3 categories: 1. Prerenal azotemia is caused by a decrease in renal blood flow, e.g. due to decreased cardiac output 2. Renal azotemia results from damage to the kidney 3. Postrenal azotemia is due to obstruction of urine flow, e.g. by prostatic hypertrophy or tumor 49 1. LABORATORY FINDINGS IN ACUTE GLOMERULONEPHRITIS Acute diffuse inflammatory changes in the glomeruli with hematuria, RBC casts, mild proteinuria, and often hypertension, edema, and azotemia A) Serum chemistries Elevated SUN Elevated creatinine Elevated uric acid SUN/creatinine > 20 Decreased creatinine clearance GFR decreased Acidosis due to retention of phosphate, sulfate, amino acids, and other metabolic acids B) Urinalysis Microscopic hematuria (smoky urine) Macroscropic hematuria (red urine) Casts: Red cell casts (blood casts) Proteinuria, mild to moderate C) Selectivity ratio The ratio of the clearances of a high and a low molecular weight protein (IgG and albumin, respectively) gives an indication of the nature of glomerular damage in glomerular proteinuria (selective vs. non-selective). Selectivity ratio = IgG clearance albumin clearance High selectivity < 0.15; e.g., minimal change glomerulonephritis Poor selectivity > 0.30; other than minimal change disease 2. LABORATORY FINDINGS IN CHRONIC GLOMERULONEPHRITIS Slowly progressive glomerular disease: the syndrome is due to several diseases of different etiology; the disease is characterized by diffuse sclerosis of glomeruli and loss of nephrons A) Serum chemistry values Uremia (elevated SUN, creatinine and uric acid) Hyponatremia Hyperkalemia Hypocalcemia Hyperphosphatemia B) Urinalysis Proteinuria Episodic hematuria C) Anemia 50 Decreased creatinine clearance Acidosis Elevated alkaline phosphatase SUN/creatinine < 10 GFR decreased Isosthenuria (Sp. Gr. fixed between 1.008 and 1.012) Cylindruria (presence of tubular casts in the urine) 3. LABORATORY FINDINGS IN NEPHROSIS (NEPHROTIC SYNDROME) Complex condition that follows prolonged increase in glomerular permeability for proteins. Triad Proteinuria, > 3.5 g/day Hypoalbuminemia Hyperlipidemia In addition, edema is generally present SUN/creatinine ~12 (normal) GFR normal The decreased oncotic pressure stimulates hepatic lipoprotein synthesis and thus, hyperlipidemia is often seen in this condition. Increase in lipoproteins is also caused by loss of factors regulating lipoprotein synthesis. In the nephrotic syndrome there is a loss of a variety of proteins, such as transferrin, cortisol-binding globulin, thyroxine-binding globulin, and some coagulation factors. However, some coagulation factors are increased, e.g. Factor V and VIII, leading to thrombosis. A) Major diagnostic findings Low albumin, 1-2.5 g/dL (normal 3.4-4.8 g/dL) Urea nitrogen normal Creatinine normal B) Less specific findings Increase in phospholipids Low ceruloplasmin A/G (albumin/globulin) ratio reversed Increase in triglycerides Increase in cholesterol and lipoproteins Low complement Low transferrin C) Urinalysis Reduced volume Large amounts of protein, usually 3.5 - 10 g/d; values up to 50 g/24 h are possible Excretion of red and white cells is common Casts: many hyaline and finely granular casts due to low urine flow Casts may contain fat (fat is doubly refractile) Oval fat bodies (epithelial cells and macrophages loaded with fat) D) Serum Thyroxine-binding globulin may be decreased; accordingly, total T4 (thyroxine) may be decreased; thyroid function, however, is normal (normal free thyroxine). Total globulin concentration may be normal, but 2-globulins are increased, and -globulins may be low (see serum protein section). 51 4. LABORATORY FINDINGS IN ACUTE PYELONEPHRITIS Acute infective tubulo-interstitial nephritis; acute pyogenic infection of the kidney - one of the most common diseases of the kidney A) Serum chemistry values Urea N, creatinine, and uric acid are normal SUN/creatinine ~12 (normal) GFR normal B) Urinalysis Pyuria (pus in urine) Microhematuria White cell casts Bacteriuria C) Hematology Leukocytosis 5. LABORATORY FINDINGS FOR DIFFERENTIATING PRERENAL AND RENAL FAILURE Table 2 Urine osmolality (mOsmol/kg) Urine: plasma ratio of urea concentration Urine: plasma ratio of osmolality Serum urea / creatinine ratio Prerenal Failure Renal Failure > 500 < 350 > 10:1 < 3:1 > 1.5:1 < 1.1:1 > 20:1 variable H. URINALYSIS The routine urinalysis is carried out in three phases: macroscopic, chemical, and microscopic analysis. 1. MACROSCOPIC EXAMINATION (GROSS EXAMINATION) Color Turbidity 2. CHEMICAL EXAMINATION 52 Specific gravity (normal, 24 hours: 1.010 - 1.025) Protein Glucose Ketone bodies Hemoglobin (occult blood) (note: myoglobin also reacts) Bile (direct or conjugated bilirubin reacts, but not unconjugated) Urobilinogen 3. MICROSCOPIC EXAMINATION Larger elements are: casts, mucous threads, parasites, ova, foreign bodies, etc. (low power) Casts, if present, are also examined under the high power field to determine their types The numbers of red cells, white cells, epithelial cells, bacteria, yeast, trichomonas, and crystals are also counted and an average count for each is recorded 4. URINE CASTS Casts are found in the urine sediment. They are formed in two ways, by precipitation and gelling of proteins in tubular fluid, and by clumping of cells in tubules. Casts are molded in the lumen of the distal renal tubules or collecting ducts. The matrix of all casts is a specific mucoprotein common to all casts, namely Tamm-Horsfall protein. The classification of casts is based on appearance, physical properties, and type of cellular components. Cells within the matrix can degenerate into coarse and finely granular casts and to waxy casts. Types of urine casts 1) Hyaline casts. The casts consist only of Tamm-Horsfall protein. They are excreted by the normal kidney in small amounts. Excretion of numerous casts is seen in all renal diseases associated with benign essential hypertension, and nephrotic syndrome. 2) White blood cell (leukocyte) casts. These casts are formed when WBC's are incorporated into the protein matrix. They enter the urine stream by ameboid movement through and between tubular epithelial cells and sometimes they cross the glomerular capillary lumen. These casts are associated with diseases with leukocytic exudation and interstitial inflammation. Example: pyelonephritis. 3) Red cell (erythrocyte) casts. Presence of these casts indicates severe injury to the glomerular basement membrane. The reddish orange color is secondary to hemoglobin pigmentation. Erythrocytes (RBC’s) are biconcave disks packed in fibrin meshwork within the cast matrix. These casts are associated with acute glomerulonephritis (most common), lupus nephritis, Goodpasture’s syndrome, and subacute bacterial endocarditis (SBE). 4) Renal epithelial casts. These casts are due to constant desquamation and renewal of the renal epithelium. Their presence points to a pathological process occurring in the kidney and affecting the tubular portion of the nephron (tubular damage). Epithelial casts are associated with exposure to nephrotoxic agents and exposure to some viruses. 5) Granular casts. These casts are formed from breakdown products of cellular casts and immunoglobulins. There is a progression from coarsely granular to finely granular casts. 6) Waxy casts. These are the result of progressive degenerative changes occurring in cellular casts and they are associated with severe chronic renal disease and amyloidosis. 53 7) Fatty casts. These casts are probably due to leakage of lipoproteins through the glomerular filter and are associated with nephrotic syndrome, diabetes mellitus, and damaged renal tubular epithelial cells. 8) Mixed cell casts. Table 3: Some Commonly Seen Urinary Crystals Crystal Calcium oxalates Sodium urates Triple phosphates (magnesium ammonium phosphate) Ammonium biurates Amorphous phosphates Tyrosine Leucine Sulfonamides Cystine Appearance “Envelopes” “Whetstones” Urine pH acid acid “Coffin lids” alkaline “Thorn apples” Amorphous debris Needles in rosettes Spheres Sheaves Hexagons alkaline alkaline acid acid acid acid Drug crystals (e.g., ampicillin needles, primidone hexagons) are formed from drugs that are present in relatively high concentrations and that are relatively insoluble in water at the urinary pH. 54 I. CASE STUDIES CASE 1 (Ann. Pharmacother. 36(9):1380-1382, 2002) A 73-year-old white man, with a medical history of non-small-cell lung cancer and idiopathic myelofibrosis with myeloid metaplasia, was prescribed levofloxacin 500 mg/d orally because of a lower urinary tract infection. Three days after starting treatment with levofloxacin, the patient was admitted to the hospital with palpable purpura and erythematous skin lesions over the lower limbs and trunk, with a markedly diminished urine output. His vital signs were BP 155/70 mmHg, HR 92 beats/min, RR 14 breaths/min, and (axillary) Temp 37.1°C. Initial patient labs (2 weeks prior to levofloxacin): creatinine 1.0 mg/dL, urea 38 mg/dL. On admission the patient’s serum creatinine concentration was 6.4 mg/dL and serum urea nitrogen 190 mg/dL. Serum electrolytes were normal. Hemoglobin and hematocrit were 8.4 g/dL and 24%. Bilirubin, aminotransferase enzymes, and alkaline phosphatase were normal; lactate dehydrogenase was also normal. Urinalysis disclosed 3+ proteinuria, with no casts or crystals, an acidic pH (5.0), and a reduced specific gravity (1.007). The daily urinary output was 0.6, 0.5, and 0.8 L over the first 3 days of the hospital stay, respectively. Cultures of peripheral blood and urine grew no pathogens. 1. How do you interpret the initial creatinine and urea results? 2. How do you interpret the admission creatinine and urea results? 3. How do you interpret the hemoglobin and hematocrit? 4. How do you interpret the bilirubin, aminotransferase and alkaline phosphatase? 5. How do you interpret the urinalysis results? 6. What is a probable mechanism for these findings? 7. What medication recommendations would you have for treating this patient? 55 CASE 2 (Clin. Infect. Dis. 36(8):1082-1085, 2003) A 49-year-old AIDS patient with multiple drug-resistant HIV gets tenofovir added to his drug regimen. His medical history was also notable for adrenal insufficiency, hypogonadism, anemia, peripheral neuropathy, asthma, and large B cell lymphoma of the thoracic spine. His serum creatinine level had ranged from 1.9 to 2.8 mg/dL, and it was 2.3 mg/dL just before he started receiving tenofovir. The patient had received enteric-coated didanosine formulation (400 mg/day) several months before tenofovir therapy was started. The patient returned for a follow-up visit 2 weeks after he started the new regimen, at which time the creatinine level was 2.0 mg/dL, bicarbonate 21 mmol/L and albumin 3.0 g/dL. Due to lower extremity edema he was prescribed furosemide. Six weeks after starting tenofovir the patient was brought to the emergency department with a 4-day history of progressive fatigue, weakness, confusion, oliguria, and myalgia. At admission to the emergency department, the patient was noted to be hypotensive (blood pressure, 90/50 mmHg), and laboratory studies included: BUN 78 mg/dL, creatinine 7.6 mg/dL, arterial blood gas pH 6.93, bicarbonate of 5 mmol/L and lactate of 5.5 mmol/L (normal 0.5-2.2 mmol/L). No evidence of infection was found with blood culture. 1. If the patient weighed 74.6 kg calculate his estimated GFR based on the creatinine of 2.3 mg/dL. 2. Based on this creatinine are dose adjustments required for tenofovir? 3. How do you interpret the lab values at the 2 week follow-up? 4. How do you interpret the lab values at the 6 week follow-up? 5. Calculate the patients estimated GFR at six weeks post therapy. 6. Discuss the importance of knowing the renal status in patients dosed with tenofovir. 7. What is this patient’s prognosis? 56 V LABORATORY TESTS OF GASTROINTESTINAL DISEASE 57 58 A. OBJECTIVES To describe laboratory tests used to diagnose malabsorption and maldigestion To describe how to interpret various laboratory tests for Helicobacter Pylori To describe the Schilling test for vitamin B12 absorption To describe the tests for diagnosis of Celiac disease To describe the sweat test for cystic fibrosis B. KEY TERMS Achlorhydria - lack of production of stomach acid Celiac disease - a malabsorption syndrome precipitated by the ingestion of gluten-containing foods Cystic fibrosis - disorder characterized by widespread dysfunction of exocrine glands, characterized by chronic pulmonary disease, pancreatic deficiency and high levels of electrolytes in sweat GERD - gastro esophageal reflux disease Zollinger-Ellison (Z-E) syndrome - triad of hypergastrinemia, peptic ulcers and gastrinsecreting tumors C. BACKGROUND/SIGNIFICANCE The GI tract is a vast and diverse system subject to a plethora of disorders. Many different laboratory tests have been developed that are invaluable tools that clinicians use to diagnosis and manage these disorders. Drug therapy is well established for the treatment of many diseases of the GI tract and it is essential that pharmacists understand how to interpret laboratory tests related to GI function in order to recommend appropriate medications. D. GERD The most common disorder in the esophagus It affects up to 10% of the population The commonly used laboratory test is based on the measurement of the amount of HCl produced by the stomach 1. GASTRIC FUNCTION TESTS Measurement of the amount of HCl produced by the stomach under basal (resting) and fasting conditions and without exposure to visual, auditory or olfactory stimuli. This is followed by testing after maximal stimulation. 59 2. THE STANDARD METHODS NOW IN USE ARE AS FOLLOWS: A) Basal gastric secretion Following a 12 hour overnight fast, the patient is intubated under fluoroscopic guidance and the residual gastric secretion is aspirated. B) Pentagastrin stimulation test Pentagastrin is a synthetic pentapeptide containing the four C-terminal amino acids of gastrin coupled to alanine. It has biologic activity similar to gastrin, stimulating HCl and pepsin secretion. Gastric Acid Young people > Old people Gastric carcinoma < Controls Men > Women Gastric ulcer < Controls Duodenal ulcer > Controls C) Clinical interpretation Achlorhydria (anacidity) after stimulation is seen in all cases of pernicious anemia, and in some patients with advanced carcinoma of the stomach. It may also be seen in a variety of other conditions, such as hypochromic anemia, aplastic anemia, hypothyroidism, nutritional megaloblastic anemia and in relatives of patients with pernicious anemia. Low values are found in gastric carcinoma, benign gastric ulcers, in females and in aging persons. Hyperacidity is seen in duodenal ulcer, but there is considerable overlap with the normal range. Extreme hyperacidity as well as high serum gastrin levels are seen in patients with Zollinger-Ellison (Z-E) syndrome. In this condition there is also a high ratio of basal/maximal acid output. 3. CAUSES OF GASTRIC DISORDERS VS. HYPERGASTREMIA Disorder Gastric acid secretion Zollinger-Ellison syndrome greatly Hypersecretion of gastrin by antral G-cells greatly Pernicious anemia Post vagotomy Chronic renal failure variable 4. DRUGS IMPORTANT IN HYPERACIDITY A) H2 antagonists (e.g., cimetidine, ranitidine) Inhibit gastric acid secretion due to histamine stimulation B) Proton-pump inhibitors (e.g., lansoprazole, omeprazole) Irreversibly inhibit pump that produces H+ (H+-K+ ATPase) Little effect on intrinsic factor, pepsin or overall volume of secretion 60 C) Antacids (e.g., Mg(OH)3, Al(OH)3, CaCO3) Neutralize HCl 5. HELICOBACTER PYLORI Helicobacter pylori has been implicated in many gastrointestinal diseases including duodenal and gastric ulcer, gastritis, and possibly gastric cancer. In asymptomatic individuals colonization by Helicobacter pylori increases with age up to 50% in the elderly. A) Diagnostic tests 1) Biopsy a) Culture b) Detection of urease enzyme activity by placing the biopsy specimen onto a substrate containing urea and monitoring change in pH. 13 2) C-breath test Helicobacter pylori is a bacterium that is capable of living in the low pH environment of the stomach; as part of its adaptation to this hostile environment, it produces high levels of urease (which can help raise the local pH). Based on urease production by Helicobacter pylori, a patient ingests a 13C-labeled urea, and the breath of the patient is monitored for the appearance of 13C-labeled CO2. Within minutes after ingesting the labeled urea, the 13C to 12C ratio of respiratory CO2 starts to increase, reflecting the addition of the 13CO2 from the labeled urea. The maximum ratio of 13C to 12C peaks at 1.5 hours and then decreases to baseline. Using the 13C methodology, detection of Helicobacter pylori infection is determined with a clinical sensitivity of 94% and a specificity of 94.7%. 3) Immunoassay for IgG antibody There is a strong correlation between serum antibody and the presence of Helicobacter pylori in culture material obtained by biopsy. However, the test may remain positive for several years, even after successful treatment. B) Preferred therapies for Helicobacter pylori infection Triple therapy includes a proton-pump inhibitor (twice a day) and plus two of the following: amoxicillin, clarithromycin or metronidazole Quadruple therapy includes a proton-pump inhibitor (twice a day), tetracycline, bismuth, and metronidazole E. MALABSORPTION/MALDIGESTION A variety of diseases causes a disturbance in digestion and absorption. Maldigestion: Dysfunction of the digestive process that can occur in a number of sites in the gastrointestinal tract. Malabsorption: Dysfunction of the absorptive process by the gut caused by gluten, inflammation, infection, surgical resection, vitamin deficiency and other factors. 61 Both conditions lead to one common syndrome called malabsorption. Thus, the diagnostic process is designed to: 1. Demonstrate the presence of malabsorption 2. Identify the type of disease process All major phases of absorption may simultaneously be affected, i.e. fats, proteins, carbohydrates, vitamins, minerals, etc. This syndrome, called general malabsorption, is characterized by amylorrhea (excess starch), steatorrhea (excess fat), and creatorrhea (meat fibers, protein). 1) GENERAL MALABSORPTION/MALDIGESTION In this syndrome all major phases of absorption may be involved. This syndrome may be due to: 1. 2. 3. 4. Pancreatic disease such as chronic pancreatitis, carcinoma, cystic fibrosis Zollinger-Ellison syndrome Liver disease with blockage of bile flow Intestinal diseases Any disease that destroys the intestinal lumen/border function (e.g., celiac disease, Crohn’s disease, etc.) 5. Resin treatment A) Tests of nonspecific biochemical abnormalities seen in malabsorption Serum calcium is usually low because of decreased absorption and decreased serum albumin to which normally 55% of the calcium in serum is bound Serum alkaline phosphatase is elevated; its source may be the gut but usually is the bone; chronically decreased vitamin D and calcium absorption lead to osteomalacia, a condition accompanied by inadequate mineralization of osteoid and elevations of serum alkaline phosphatase Serum urea nitrogen usually is low due to the decreased protein absorption Hypocholesterolemia is seen in the full syndrome of malabsorption, presumably due to decreased absorption of lipids and decreased synthesis of cholesterol The prothrombin time may be markedly prolonged, and the patient may even present with hemorrhages; a combination of reduced vitamin K absorption and decreased synthesis of clotting factor due to protein deficiency or decreased liver synthesis is responsible The glucose tolerance curve often is flat because of defective glucose absorption 62 B) Specific malabsorption/maldigestion defects Disaccharidase deficiencies: o lactase deficiency o sucrase deficiency o maltase deficiency Glucose-galactose malabsorption Pernicious anemia Protein-losing enteropathy Blind-loop syndrome Jejunal diverticulum Parasitic infestations C) Tests used in the evaluation of malabsorption/maldigestion 1) Carbohydrate malabsorption D-xylose absorption test (decreased) Disaccharidase test (decreased) Breath hydrogen test (increased) 2) Fat malabsorption Fecal fat determination (elevated) 14C-triolein breath test (decreased) 3) Bacterial overgrowth 14C-Xylose breath test (increased) 4) Others Schilling test (decreased) Sweat test (increased in cystic fibrosis) D) Celiac disease Celiac disease (gluten-sensitive enteropathy) results from an immune-mediated intolerance to ingested wheat gluten or related proteins from rye and barley Presents in varying degrees of severity, from abdominal discomfort, diarrhea, flatulence, bloating, steatorrhea, weight loss, fatigue and malaise to subacute, silent or latent celiac disease The prevalence is estimated at about 1% in North America and Europe More than 90% of patients with celiac disease have HLA-DQ2 and essentially all the remaining patients have HLA-DQ8 Celiac disease can be triggered after surgery, pregnancy, emotional stress or viral infections; of particular high risk are those individuals with other autoimmune diseases Diagnosing celiac disease can be difficult because some of its symptoms are similar to other illnesses such as Crohn’s disease, ulcerative colitis, diverticulosis, intestinal infections, chronic fatigue syndrome and depression 63 Diagnosis Accurate diagnosis of celiac disease requires gluten in the patient’s diet at the time of testing The initial test is detection of IgA endomysial antibody (EMA) against tissue transglutaminase; this test is close to 100 % sensitive with a specificity of around 95% If this test is positive, the patient should have multiple small bowel biopsies from the second part of the duodenum and beyond to establish the diagnosis The diagnosis is then confirmed by a resolution of symptoms following the introduction and maintenance of a strictly gluten-free diet In those patients that appear to have celiac disease but don’t have an elevated EMA, consider that 2% of patients with celiac disease will be IgA deficient and unable to make IgA antibodies; a total IgA quantitation is indicated in these cases; these patients, and patients under the age of 3, should be tested for IgG antibodies to tissue transglutaminase Since celiac disease is a relatively common disease associated with long-term complications that are treatable by a gluten-free diet, it has been considered for public-health screening Treatment The only treatment for celiac disease is a lifelong gluten-free diet 2. GASTRIC AND ILEAL FUNCTION Schilling Test - absorption of vitamin B12 Vitamin B12 (cobalamin) is an essential cofactor for DNA synthesis. It requires gastric synthesis of intrinsic factor and proper ileal function for its absorption. B12 deficiency can be due to decreased intrinsic factor or decreased absorption due to pancreatic or ileal disease. The Schilling test is performed by orally administering 57Co-radiolabeled B12 and quantitating its appearance in the serum, feces or (most commonly) in the urine. A reference population excretes > 8% of the ingested dose in a 24 hour urine collection while < 7% is excreted in pernicious anemia. If the abnormality corrects with the coadministration of intrinsic factor with B12, the defect is due to deficiency of intrinsic factor rather than malabsorption due to pancreatic or ileal causes. 3. SWEAT TEST FOR THE DIAGNOSIS OF CYSTIC FIBROSIS This is the most reliable laboratory test for the diagnosis of cystic fibrosis. In cystic fibrosis, sweat chloride values are 60-120 mmol/L; normal values are < 60 mmol/L. The test becomes positive within3 to 5 weeks of age. Only 1 to 2 % of affected patients have sweat chloride values below 60 mmol/L and only 1 in 1000 has values below 50 mmol/L. Positive tests are observed in a number of other diseases; however, those are rare and are generally clinically distinct from cystic fibrosis. 64 The Cystic Fibrosis Foundation of the U.S. accepts only the sweat test done by iontophoresis with direct determination of chloride or sodium. Use of ion-specific electrodes applied directly to the skin or agar plates for the determination of Cl- is no longer accepted. A) Collection of sample A 0.3% solution of pilocarpine (a cholinergic drug) is introduced into the skin by iontophoresis to induce sweating. Sweat is collected with a gauze pad, weighed, eluted, and analyzed for Cl- and less often Na+. B) Test for sweat chloride and sodium Gauze pad is weighed, eluted with measured amount of distilled water, and electrolytes are determined. C) Normal values Chloride: < 60 mmol/L Sodium: 10-90 mmol/L D) Elevated values are indicative of cystic fibrosis Chloride 60-120 mmol/L (no overlap of normal) Sodium 60-180 mmol/L (overlaps normal; less useful) F. DETECTION OF OCCULT BLOOD (HEME) PRINCIPLE The detection of blood in feces is an important diagnostic aid in establishing the occurrence of internal bleeding resulting from gastrointestinal malignant growths or from gastric and duodenal ulcers. Severe gastrointestinal bleeding (60-90 mL blood per day) is visually recognizable because "tarry" (black) stools are excreted and further microscopic examination of these stools reveals the presence of erythrocytes. In those instances where much smaller quantities of blood are not visually or microscopically apparent, more sensitive techniques are required to detect this hidden, or so-called "occult," blood. Because heme-containing substances possess peroxidase activity, this property can be used to detect the presence of occult blood in feces or urine. Colorless, aromatic substances (such as guaiacol, o-tolidine, di-orthoanisidine) are catalytically oxidized to blue chromogens by heme in the presence of H2O2 when the reaction is performed in an acid medium. The sensitivity of the test is adjusted in such a way that a physiologic amount of blood loss will not give a positive reaction. To establish that occult blood in feces is due to gastrointestinal hemorrhages, other possible sources of blood from hemorrhoids, menstrual flow, ingested blood derived from the nose or mouth, and perianal bleeding must be excluded. In addition, ingested meat contains hemoglobin and myoglobin and the presence of heme in stool derived from these foodstuffs may give false positive tests for fecal occult blood. Thus, for best results patients should be placed on meat-free or low-meat diets for three days before evaluation for occult blood in their stools. Normally, up to 2.5 mL of blood may be lost daily in the stools. Drugs (such as salicylates, steroids, reserpine, indomethacin, colchicine, iron) 65 often cause increased gastrointestinal blood loss, resulting in positive occult blood tests. Potent reducing agents such as ascorbic acid may quench the reaction (false negative). False positives may be seen following ingestion of horseradish (peroxidase). To reduce the number of false negative results, the test should be requested on several different occasions. 66 G. CASE STUDIES CASE 1 The patient is a 48-year old male with chief complaint of chronic diarrhea and epigastic pain for the past year. He states that the diarrhea is watery and without blood, pus or mucus. He has a history of duodenal ulcer for which he takes cimetidine. He has been treated for H. Pylori without improvement of his symptoms. He reports a 25-pound weight loss over the past 6 months. He denies use of laxatives. PMH: None Vitals: T 98, P 88, BP 114/55, R 28, wt 200 Physical exam: Labs: Gen: no acute distress EENT: PERRLA, EOMI (extra ocular muscles intact) Respiratory: Clear to auscultation Cardiac: RRR (rate rhythm regular) Abdomen: bowel sounds present, abdomen soft, no organomegaly Extremities: No cyanosis, clubbing, or edema Neurologic: Alert and oriented, 5/5 strength, sensation intact Rectal: guiac negative, normal sphincter tone Na 137, K 3.3, Cl 96, Bicarb 27, Bun 18, Creat 0.8, Glucose 167 Additional studies reveal: Gastrin: 1482 pg/mL (reference 0-100 pg/mL) Colonoscopy with biopsies: normal Stool fat: negative O&P: negative Stool culture for pathogenic bacteria: negative Based the high gastrin levels an octreotide scan was ordered which showed intense octreotide activity arising from the distal second part of the duodenum. 1. What is your diagnosis? 2. What is the recommended treatment? 3. This patient is at increased risk of having what syndrome? 67 CASE 2 (J. Athl. Train. 40(4):360-364, 2005) A 20-year-old student athlete female (height, 183 cm; weight, 81 kg) presented with symptoms suggestive of the early stages of an eating disorder shortly after beginning college. An eating disorder was suspected based on a rapid decrease in body mass (8.1 kg in 20 days), loss of appetite, diarrhea, and vomiting after meals. On initial referral, the athlete’s physical examination revealed a body mass of 72.9 kg with 14.5% body fat, with other relevant findings of diarrhea, fatigue, bloating, and abdominal pain. The results of a routine complete blood count are shown below. 1. What is the differential diagnosis? 2. What additional tests would be indicated? 3. What are the characteristic biopsy findings that would suggest Celiac disease? 4. Is this a common type of presentation for this type of disease? 5. Would a diagnosis of Celiac disease cause the observed CBC findings? 6. How would you treat this patient? 68 VI LABORATORY DIAGNOSIS OF PANCREATIC DISEASE 69 70 A. OBJECTIVES To list the functions of the pancreas To describe the structure and function of the pancreas To list complications of pancreatic disease To describe the laboratory tests used to diagnose pancreatic disease To state the functions of the various pancreatic enzymes B. KEY TERMS Acute pancreatitis - diffuse enzymatic destruction of the pancreas Amylase - an enzyme monitored for pancreatic damage; hydrolyzes polysaccharides into simple sugars Chronic pancreatitis - chronic inflammation of the pancreas, generally irreversible Lipase - an enzyme monitored for pancreatic damage; cleaves fatty acids from triglycerides C. BACKGROUND SIGNIFICANCE Acute pancreatitis occurs in 1 in 500 acute admissions and severe attacks have a 20-50% mortality rate. Most cases occur in older patients (greater than 50 years old) and are frequently associated alcoholism, hyperlipidemia and biliary disease. However, approximately 2% of acute pancreatitis cases are drug-induced. The laboratory plays an essential role in the rapid diagnosis of acute pancreatitis allowing clinicians to make appropriate therapeutic decisions. D. PANCREATIC OVERVIEW 1. PANCREATIC FUNCTIONS Secretes all major digestive enzymes (lipase, amylase and trypsin) Exocrine secretion controlled by secretin and CCK Secretes 800-3000 mL of bicarbonate rich fluid (pH 8 to 8.3) per 24 hours Endocrine functions include secretion of insulin and glucagon 2. COMPLICATIONS OF ACUTE PANCREATITIS Pseudocyst formation Ascites fluid Pleural effusion Abscess formation 71 E. LABORATORY FINDINGS In the diagnosis of acute pancreatitis, the following tests are diagnostically most useful: Serum amylase and isoenzymes Serum lipase Reference Range 28-85 U/L 20-250 U/L Acute pancreatitis often is accompanied by changes in other biochemical parameters which are less specific: Serum calcium Serum methemalbumin Plasma glucose Serum bilirubin Triglycerides Figure 1: Anatomy of Pancreas 1. SERUM AMYLASE A small amount of pancreatic amylase normally escapes into the circulating blood and, having a molecular weight of only about 45,000, is readily excreted by the kidney into the urine. Thus, increased entry into the blood or decreased renal excretion generally lead to increased blood levels. 72 A few hours after the onset of acute pancreatitis, serum amylase levels become elevated, but return to normal levels within two to three days. Elevations >5× the upper reference limit (URL) are considered pathognomonic for pancreatitis. Amylase also is found in other tissues, especially in the salivary glands and Fallopian tubes (may cause elevated serum amylase in ectopic pregnancy), but amylase activity to a lesser extent is also present in many other tissues (liver, muscle, adipose tissue, kidney, brain, lung, intestine, spleen, serous ovarian tumors, some lung tumors and most body fluids and excretions). However, under normal conditions 50% of the circulating blood amylase is derived from the pancreas. The clinical specificity of this test is low. In contrast to some other serum enzymes, serum amylase is relatively stable; even at room temperature it is stable for several days. 2. AMYLASE AND ISOENZYMES The isoenzymes of amylase are S (salivary) and P (pancreatic). They can easily be measured immunochemically; use of the P-isoenzyme increases specificity for acute pancreatitis. 3. MACROAMYLASEMIA This is a benign condition in which normal amylase forms macromolecular complexes with immunoglobulins (IgG, IgM) or it exists as a large polymeric aggregate, resulting in a variety of “macroamylases,” which, because of their large molecular size, are not excreted by the kidney. This results in elevated amylase levels in the serum. This condition has been observed following treatment with hog pancreatic extracts and occasionally in malabsorption syndromes, but otherwise is usually innocuous. Its clinical significance lies in the fact that the hyperamylasemia or macroamylasemia may lead to misinterpretations in the differential diagnosis of abdominal distress. Macroamylasemia accounts for 2.5% of hyperamylasemic conditions. One per cent of healthy subjects have macroamylasemia. It requires no treatment and, in fact, may be transient. In this condition, the amylase/creatinine clearance ratio described below is usually <1% (normal 1-5%), and the urine amylase is usually low. 4. SERUM LIPASE Serum lipase activity is due to a number of enzymes from various sources, predominantly the pancreas. Lipases are reabsorbed by the kidney, and lipase activity is undetectable in the urine. Serum lipase levels due to pancreatic disorders closely parallel the changes of serum amylase except that serum lipase may remain elevated longer than amylase (up to 2 weeks). Elevated serum amylase and lipase are strong evidence for a pancreatic process. When the serum amylase is elevated and the lipase is normal, non-pancreatic causes of the hyperamylasemia are more likely. (Note: lipase increases of 3-5 times the upper reference limit are pathognomonic for pancreatitis.) 73 Increases of serum lipase are seen in: Acute pancreatitis Carcinoma of the head of the pancreas Alcoholic cirrhosis Severe azotemia (renal) Trauma to adipose tissue Fat embolism Hyperalimentation therapy - induces lipase activity 5. URINARY AMYLASE Reference Range: 1-20 U/h or 20-480 U/H24h Renal excretion of amylase depends on the serum levels, and in hyperamylasemia increased amounts of amylase appear in the urine. Since this excretion is relatively rapid following moderate bouts of pancreatitis, serum levels may be borderline or normal while urinary amylase may be elevated. A urinary amylase determination is most useful in: 1. Macroamylasemia, where the urinary values are normal or low. 2. Patients who present with the clinical picture of pancreatitis while their serum amylase is normal. Renal Clearance of Amylase Reference Range for amylase/creatinine clearance: 1-5% In pancreatitis, values range from 5% to greater than 20% In macroamylasemia, values are usually less than 1% The renal clearance of amylase has been shown to range from 1 to 3 mL/min and is constant over a wide range of urine flow. Increased blood levels of amylase are followed by increased excretion into the urine. It has been shown that this increase in excretion in pancreatitis is further augmented by an increased renal clearance rate of amylase (see urinary amylase). The test can be very useful in the confirmation of macroamylasemia. In order to correct for the interindividual differences in renal function, it has been found advantageous to express amylase clearance as a percentage of creatinine clearance: the so-called amylase/creatinine clearance ratio. Urine Amylase × Urine Volume per unit of time Amylase Clearance Serum Amylase = × 100% Creatinine Clearance Urine Creatinine × Urine Volume per unit of time Serum Creatinine = 74 Urine Amylase Serum Amylase × Serum Creatinine Urine Creatinine × 100% Notice that the factors of urinary volume and time cancel out of the equation which eliminates the need for cumbersome, timed urinary and blood collections. The test thus can be carried out simply on simultaneously collected random samples of blood and urine. Increased clearance is not a specific response to pancreatitis, but instead may be due to competition for reabsorption of low-molecular-weight proteins by the renal tubules. Increases in amylase clearance and the ratio of amylase to creatinine clearance are observed in a number of non-pancreatic disorders, such as burns, myeloma, duodenal perforations, and following extraperitoneal surgical procedures. Comparisons of serum and urine tests have shown that serum enzyme tests had greater diagnostic utility than urine tests. 6. OTHER BIOCHEMICAL MARKERS A) Serum calcium The hypocalcemia of pancreatitis is a complex phenomenon involving saponification as well as glucagon and gastrin release from the pancreas which in turn, stimulates calcitonin release from the thyroid, leading to phosphaturia and calciuria. Since 50% of calcium is protein bound, hypoalbuminemia will further depress calcium levels. Calcium remains low despite calcium infusions. There appears to be a correlation between the degree of hypocalcemia and the clinical severity of the pancreatitis with calcium levels below 7 mg/dL constituting a very ominous sign as they are associated with a high percentage of fatal outcomes. It is of interest to mention here that in the acute “pancreatitis of hyperparathyroidism” the hypocalcemia does not occur, indicating the importance of the roles of hormones in controlling the calcium blood levels in pancreatitis. B) Triglycerides Triglycerides may be markedly elevated in acute pancreatitis often causing turbidity of the serum. This is also considered an ominous sign. Note that hyperlipidemia can be a cause of pancreatitis. C) Plasma glucose Acute pancreatitis often leads to glucose intolerance, with transient hyperglycemia and abnormal glucose tolerance curves (“burnt out” pancreas). F. CHRONIC PANCREATITIS In chronic pancreatitis the biochemical changes are quite variable depending on the severity and stage of the disease. For instance, serum amylase may be elevated, normal or even decreased. With long-standing pancreatitis, sufficient pancreatic destruction may have occurred so that amylase cannot be elaborated. Duodenal intubations often are necessary to establish the diagnosis. A more detailed discussion here is beyond the purview of this chapter. 75 G. CASE STUDY CASE 1 (Ann. Pharmacother. 36(12):1883-1886, 2002) A 33-year-old woman was admitted to the hospital with severe and constant upper abdominal pain (pain level score 8/10) associated with nausea. She had been diagnosed with primary hypertension and was treated with felodipine without adverse effects. Because of inadequate control of her blood pressure 10 days before admission, felodipine was discontinued and irbesartan 300 mg and hydrochlorothiazide 12.5 mg once daily was started. She denied recent alcohol intake, alcoholism, or recent viral infection. There was no personal or family history of pancreatitis, cholelithiasis, or hyperlipidemia. Examination revealed an obese (weight 107 kg, height 169 cm) woman with low-grade fever (37.4ºC), relative hypotension (116/55 mmHg), and moderate upper abdominal tenderness with no bowel sounds. Laboratory data showed increased serum concentrations of lipase and amylase; all other hematologic and biochemical variables were normal (Table 1). Table 1: Lab Values on Admission Lab Value Alanine aminotransferase (U/L) 25 Alkaline phosphatase (U/L) 82 Amylase (U/L) 152 Bicarbonate (mEq/L) 26 Bilirubin, total (mg/dL) 0.8 Creatinine (mg/dL) 0.66 Glucose (mg/dL) 92 Hemoglobin (mg/dL) 13.6 Lipase (U/L) 1014 Oxygen, arterial (mmHg) 84 pH, arterial 7.39 Potassium (mEq/L) 4.2 Sodium (mEq/L) 139 Triglycerides (mg/dL) 87 3 3 White blood count (×10 /mm ) 11.5 Reference Range 5-55 20-110 20-110 22-26 0.12-1.17 0.45-1.02 63-108 11.5-16.0 30-300 75-100 7.34-7.44 3.5-5.0 137-145 35-155 4.0-11.0 1. How do you interpret these lab values? 2. Are patient’s symptoms and clinical presentation consistent with the laboratory values? 3. What is the appropriate course of action for this patient? 4. Is this a common ADR for this drug combination? 76 VII LABORATORY DIAGNOSIS OF PROTEIN ABNORMALITIES 77 78 A. OBJECTIVES To understand how electrophoresis is used to aid in the diagnosis of various disease states To learn how to interpret electrophoresis patterns To understand how immunofixation electrophoresis is used to identify the type of immunoglobulin present B. KEY TERMS Bence Jones protein - free light chains Congenital - existing at birth Monoclonal immunoglobulinopathies - increased concentration of a single immunoglobin that originates from a single plasma cell clone; also known as M-proteins MGUS - monoclonal gammopathy of undetermined significance Paraprotein - abnormal protein appearing in large quantities as a result of a pathological process Polyclonal hyperimmunoglobulinemias - increased concentration of immunoglobulins from many different plasma cell lines C. BACKGROUND/SIGNIFICANCE Understanding how to interpret concentrations of proteins in body fluids is important because proteins have crucial roles in many biological responses. In addition, changes in protein concentrations often provide the initial evidence allowing clinicians to diagnose a variety of disease states. For pharmacists, understanding the analysis and interpretation of plasma proteins is especially important as most plasma proteins are synthesized by the liver which is a frequent target of drug toxicity. D. ANALYTICAL APPROACH Serum protein electrophoresis: electrophoresis is a screening test which can be followed by quantitative assay of specific proteins; independent measurement of total protein is required for conversion to absolute amounts Identify abnormalities in immunoglobulin fraction by serum immunofixation Follow specific protein abnormalities by quantitative analysis of the protein by immunoassay ACUTE-PHASE REACTANTS Tissue damage and subsequent inflammation caused by trauma, tissue necrosis, immunomediated cell damage (as in auto-immune or immune complex disease), as well as viral and bacterial infections trigger the acute phase response which includes the increased synthesis of the acute phase proteins. In the case of bacterial infection the response is much larger than that by an equivalent amount of inflammation of other 79 origin. The difference is due to the potent effect of bacterial endotoxin in inducing cytokine release from macrophages, which in turn stimulates the liver to synthesize acute phase proteins. The time curve of elevation of individual acute phase proteins and the degree of elevation of individual proteins differs in various disease entities. The alteration in acute phase protein concentration in serum is due mainly to changes in the transcription rate of genes within hepatocytes. The specificity of increases in acute phase proteins is high for tissue injury but low for specific disease entities. One of the most useful aspects of acute phase protein measurements is the sensitivity to small amounts of inflammation. C-reactive protein (CRP) is the measurement of choice for the detection of acute inflammation and in monitoring the response to treatment of inflammatory disease. In chronic conditions, the erythrocyte sedimentation rate may be the preferred measurement. IMPORTANT ACUTE PHASE PROTEINS A) α1-globulins 1) α1-antitrypsin serine protease key role in elasticity of lungs decreased concentrations cause liver disease and hepatocellular damage hereditary deficiency leads to emphysema and liver disease 2) α1-acid glycoprotein (orosomucoid) binding protein for many drugs B) α2-globulins 1) Haptoglobin binds free hemoglobin naturally bacteriostatic monitored in hemolytic disease (e.g., haptoglobin ↓ in hemolytic disease. Haptoglobin binds heme and is then removed from circulation) 2) Ceruloplasmin copper-binding protein essential role in mechanism for storing Fe in ferritin C) γ-globulin CRP most sensitive acute phase reactant activates complement recognizes potentially toxic autogenous (from self) compounds released from damaged tissue can be used to follow progression of disease “high sensitivity” CRP is used as a prognostic indicator of future adverse cardiac events 80 In the acute phase response the most striking pattern on electrophoresis is an increase in the α1 and α2 regions. Albumin, prealbumin and transferrin are negative acute phase reactants (decrease in concentration) thus there may be little change in the total protein concentration. E. GENERAL COMMENTS ON CLINICAL ABNORMALITIES OF GAMMAGLOBULINS 1. CONGENITAL DEFICIENCY Immunodeficiency syndromes that are present at birth 2. SECONDARY HYPOGAMMAGLOBULINEMIAS Protein-losing conditions Malignant lymphomas Leukemias Multiple myeloma 3. POLYCLONAL HYPERIMMUNOGLOBULINEMIAS Chronic liver disease Chronic infections Malignancies Autoimmune diseases Miscellaneous conditions 4. MONOCLONAL IMMUNOGLOBULINOPATHIES (M-PROTEINEMIA) Multiple myeloma Primary macroglobulinemia (Waldenstrøm’s) Monoclonal gammopathy of undetermined significance (MGUS) Miscellaneous conditions A single clone of immunoglobulin-producing cells usually synthesizes only a single immunoglobulin, or monoclonal protein, which separates electrophoretically as a homogeneous band giving a discrete peak. These M-proteins may be of the IgG, IgA, IgM, or (less commonly) IgD type and may appear as a sharp peak on electrophoresis anywhere within the beta and gamma regions of the electropherogram and rarely in the -region (1-2%). A) Multiple myeloma Multiple myeloma is a disease of older adults characterized by bone pain that is caused by a malignancy of plasma cells. In multiple myeloma bone marrow cells become replaced by plasma cells which often produce unregulated amounts of a monoclonal antibody that can be detected in serum. On bone biopsy plasma cells >30%, 3 or more lytic lesions would define the patient as having multiple myeloma. As bone marrow is replaced, patients become anemic and eventually have bone marrow failure. The bone marrow lesions can be visualize on x-ray as characteristic “punched out lesions” which leads to bone pain, osteoporosis, pathologic fractures, and hypercalcemia. 81 B) Waldenstrøm’s macroglobulinemia Waldenstrøm’s macroglobulinemia is characterized by IgM monoclonal proteins which cause hyperviscosity of the patients serum. Typically there is an absence of lytic bone disease and no bone tenderness. The abnormal protein is of the IgM type with a molecular weight of about 1,000,000 and a sedimentation constant of 18S (Svedberg unit). Bence-Jones proteinuria occurs in about 10% of these patients. The blood may be very viscous, because these large molecules do not readily leave the plasma for the extra cellular space. C) Monoclonal gammopathy of undetermined significance The term “monoclonal gammopathy of undetermined significance” (MGUS) denotes the presence of an M-protein in persons without clinical evidence of multiple myeloma (MM), macroglobulinemia, primary amyloidosis (AL) or other related disorders. This occurs in 50-60% of patients presenting with M-proteins. The term “benign monoclonal gammopathy” is misleading because one does not know if the process will remain stable and benign or will develop into symptomatic MM, macroglobulinemia, or a related disorder. The frequency of monoclonal gammopathies increases with advancing age. The prevalence of MGUS is 1% of patients older than 50 years, 3% of those older than 70 years, and increases up to 10% in persons over the age of 80 years. The incidence of MGUS is higher in African-Americans and lower in elderly Japanese. MGUS is characterized by: the presence of less than 3 g/dL M-protein in serum; fewer than 10% plasma cells in the bone marrow; lack of, or only small amounts of M-protein in the urine, and a low plasma-cell labeling index (PCLI). PCLI measures the synthesis of DNA and when elevated is good evidence that the patient has MM or will soon develop it. In addition, evidence of bone lesions, anemia, hypercalcemia or renal insufficiency must be absent. Most importantly, the M-protein must remain stable and no other abnormalities develop. In patients recently diagnosed with MGUS, serum protein electrophoresis should be stable. The test should be repeated in 3 to 6 months. If the M-protein is constant, the test should be repeated in 6 to 12 months. If the M-protein remains constant, electrophoresis and clinical evaluation should be performed annually thereafter. Patients should be told that the actuarial risk of malignant transformation is 17% at 10 years and 33% at 20 years. The rate of development of serious disease does not differ whether the M- protein is IgG, IgA, or IgM. However, patients should also be aware that evolution from MGUS to MM can be abrupt; and therefore, they should be advised to seek medical evaluation if symptoms develop. F. IMMUNOFIXATION ELECTROPHORESIS Example of identification of a monoclonal serum protein by immunofixation electrophoresis (Figure 1). The first track is serum protein electrophoresis (ELP). The next tracks are IgG (G), IgA (A), IgM (M), kappa light chains (K) and lambda light chains (L). The arrow indicates the position of the monoclonal protein. The second track identifies the protein as 82 IgG after reaction with IgG antibody and protein staining. The sixth track identifies the light chain as lambda. ELP G A M K L Figure 1 Example of identification of a monoclonal serum protein by immunofixation electrophoresis (Figure 2). The first track is serum protein electrophoresis (ELP). The next tracks are IgG (G), IgA (A), IgM (M), kappa light chains (K) and lambda light chains (L). The arrow indicates the position of the monoclonal protein. The third track identifies the protein as IgM after reaction with IgM antibody and protein staining. The sixth track identifies the light chain as lambda. ELP G A M K L Figure 2 83 G. ELECTROPHORETIC PATTERNS IN HEALTH AND DISEASE 84 5. α1-Antitrypsin deficiency. Phenotyping studies are suggested for confirmation. The increase in the α2fraction suggests an acute inflammatory response. 7. Pattern of nephrotic syndrome with marked decrease in albumin and increase in α 2-globulin (α 2-macroglobulin and low-density lipoprotein). Urine shows increase in all fractions except the α 2-fraction. 6. A-gammaglobulinemia, suggestive of an immunodeficiency state. 8. Pattern seen in cirrhosis, with decrease in albumin and diffuse increase in immunoglobulins involving both IgA (β-γ region) and IgG (γ region). 85 86 H. CASE STUDIES CASE 1 An 84-year-old woman was admitted to a local hospital for sudden onset of severe pain in her right clavicular region. Previously, the patient had been in good health. Physical examination was essentially unremarkable with the exception of marked tenderness over her right clavicle. The patient denies having back pain. PA and lateral chest x-rays demonstrated a fracture of the right clavicle and a compression fracture of a lower thoracic vertebra. There was a lucency on the mid-portion of the 7th left posterior rib. Skeletal exam showed multiple cortical and medullary “punched out” lesions throughout the skeleton. Laboratory: Hb 10.2 g/dL, Hct 31%, WBC 5,200/L (54 segs, 10 bands, 33 lymphs, 1 basophil, 2 eosinophils). UA: Sp. gr. 1.021, protein 3+, 3-6 WBC/HPF, 8-10 RBC/HPF SPEP: TP 10.4, Alb. 2.6, 1 0.6, 2 1.2, 0.7, 5.3 g/dL 1. What is the most probable explanation (diagnosis) for these findings? 2. What therapeutic options are available for this patient? 87 CASE 2 A 66-year-old man was admitted for biopsy of a neck mass. He had been well and actively employed as a painter until one year earlier when he fell from a ladder and sustained a subdural hematoma which was evacuated surgically. Fluid reaccumulated at the hematoma site, and a ventriculoperitoneal shunt was inserted. The shunt required multiple revisions. Infection was suspected but not documented. Four months earlier a right paratracheal mass was noted on chest x-ray and one month earlier a right supraclavicular mass was felt. On physical examination the patient had a mass on the right side of his neck, a palpable nodule in the right lobe of his thyroid, and several matted right supraclavicular lymph nodes. Laboratory: the patient had a hematocrit of 28% and hemoglobin of 9.1 g/dL. His WBC was 3300/L. The peripheral blood smear showed marked rouleaux formation of red cells, and his ESR was 147 mm/h. Serum viscosity was 5.0. Urinalysis revealed trace protein. SUN was 23 mg/dL and serum creatinine was 1.2 mg/dL. Thyroid scan demonstrated iodine uptake into a nodule which was found to be a follicular adenoma on biopsy. Biopsy of the neck mass revealed a lymphoma. SPEP: TP 8.3, alb. 2.9, 1 0.4, 2 1.1, 0.7, 3.2 g/dL 1. What is the most probable explanation (diagnosis) for these findings? 88 CASE 3 A 37-year-old woman was admitted to the hospital because of weakness and backache. For four years she has had episodic polyarticular arthritis involving her hands, knees, ankles, wrists, elbows, neck and sternal manubrium. Her arthritic exacerbations had been controlled with phenylbutazone, but the patient discontinued this medication for fear of side effects. Physical examination revealed a large effusion in the patient’s right elbow, a large effusion in her left knee with right knee tenderness, right ankle swelling and bilateral tenderness of her knuckles. The clinical diagnosis was ankylosing spondylitis. Laboratory: there was mild anemia with a hematocrit of 35% and a hemoglobin of 11.6 g/dL. The WBC was normal with an increased number of lymphocytes. The ESR was 75 mm/h. SPEP: TP 6.8, alb. 3.4, 1 0.4, 2 0.8, 0.9, 1.3 g/dL 1. What is the most probable explanation (diagnosis) for these findings? 89 CASE 4 A 49-year-old woman entered the hospital because of shortness of breath and distended abdomen. She is a chronic alcoholic who has been admitted several times earlier because of massive ascites. Her abdominal size has increased markedly over the past 2 months, and she is unable to walk more than 1/2 block because of shortness of breath. Two weeks earlier she fell, striking her back on the left side and has since had pain on the left side of her chest wall, which impairs deep inspiration. She has been following a low sodium diet and takes aldactone and vitamins. Physical examination revealed ecchymoses on the left posterior chest with tenderness in the region of the seventh to tenth ribs. Breath sounds were decreased at the left base. She had abdominal ascites with a positive fluid wave and a prominent venous pattern over the surface of the abdomen. There was 3+ edema from ankles to abdomen. Laboratory: serum electrolytes were Na+ 127 mmol/L, K+ 3.7 mmol/L, Cl- 107 mmol/L, HCO-3 19 mmol/L, and calcium 7.8 mg/dL. Serum amylase was 135 U/L and CK 96 U/L. Her WBC was 18,300/L with segs 73, bands 4, lymphs 15 and monos 7. The platelet count was 142,000/L. The ESR was 54 mm/h. Clotting studies showed PT 16.6/11.1 sec and PTT 38.3/31.1 sec. ABG’s on room air were pO2 54 mmHg, pCO2 28 mmHg, and pH 7.51. SPEP: TP 8.0, alb. 2.6, 1 0.3, 2 0.5, 0.9, 3.7 g/dL 1. What is the most probable explanation (diagnosis) for these findings? 90 VIII LABORATORY DIAGNOSIS OF DIABETES MELLITUS 91 92 A. OBJECTIVES To describe the regulation of blood glucose To describe the differences between type 1 and type 2 diabetes mellitus To understand the analysis of blood glucose To identify appropriate laboratory tests for the diagnosis of diabetes To identify appropriate laboratory tests for monitoring diabetic therapy B. KEY TERMS Hemoglobin A1c - hemoglobin that has been glycated on the terminal valine of the beta chain Glycated hemoglobin - hemoglobin that has been glycated at one or more amino acid residues Glycation - the non enzymatic covalent addition of glucose to amino groups of proteins Gluconeogenesis - formation of glucose from molecules that are not themselves carbohydrates (e.g., amino acids, lactate and glycerol) Glycogenolysis - the breakdown of glycogen to form glucose C. BACKGROUND AND SIGNIFICANCE Diabetes mellitus is a complex group of metabolic diseases characterized by under-utilization of glucose leading to hyperglycemia. There are an estimated 1.5 million Americans with type 1 diabetes and 15 million Americans with type 2 diabetes. The laboratory diagnosis of diabetes is critical as it is estimated that only half of the patients with diabetes have been diagnosed and that most patients have diabetes for 7 years prior to diagnosis. Diabetes is the 4th leading cause of death in America. Table 1: Hormonal Regulation of Blood Glucose Levels Glucose Insulin (Glucose transport across cell membranes, glucose metabolism, glycogen synthesis, lipogenesis) Glucose Growth hormone (insulin antagonist) ACTH ( glucocorticoids) Glucocorticoids ( gluconeogenesis) Epinephrine ( glycogenolysis + gluconeogenesis) Glucagon ( glycogenolysis + gluconeogenesis) Thyroxine ( glycogenolysis + gluconeogenesis) Somatostatin ( insulin secretion) Diabetes mellitus may occur from insufficient production of the insulin (injury and destruction of pancreatic islet β-cells) (type 1 diabetes) or from resistance of target tissues to the full activity of insulin (type 2 diabetes).8 It should, however, be remembered that 8 Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 26:S5-S20, 2003 93 excessive production of the hormones that drive metabolic pathways of glucose production may also result in a diabetic picture. The correct diagnosis is essential for proper drug therapy. The hormonal regulation of blood glucose levels is summarized in Table 1. D. CLASSIFICATION OF DIABETES MELLITUS (NATIONAL DIABETES DATA GROUP) 1. TYPE 1 DIABETES MELLITUS Formerly known as “juvenile onset diabetes mellitus” and “insulin dependent” Caused by pancreatic islet β-cell destruction A) Laboratory findings Elevated fasting glucose levels (> 126 mg/dL) Glycosuria Ketonuria and ketonemia frequently present Insulinopenia Elevated glycated hemoglobin Islet cell antibodies, mostly found within the first year after diagnosis Increased prevalence of the HLA antigens DR3 and DR4 B) Clinical findings Proneness to ketosis Lifelong dependence on injected insulin for control of ketosis and preservation of life Frequent onset at young age (peak at around puberty) Positive family history (variable) Possible association with failure of other endocrine organs on an autoimmune basis (hypothyroidism, hypoadrenalism) 2. TYPE 2 DIABETES MELLITUS Formerly known as adult onset diabetes mellitus or non-insulin dependent Results from insulin resistance A) Laboratory findings Elevated fasting glucose levels (> 126 mg/dL) Abnormal glucose tolerance test Variable glycosuria Elevated glycated hemoglobin Variable levels of insulin Decreased number of insulin receptors on various tissues 94 B) Clinical findings Generally ketosis-resistant; however, ketosis may develop under special stress conditions Resistance to the action of insulin Onset more frequently after 40 years of age Often associated with obesity Strong family history 3. CATEGORIES OF INCREASES RISK FOR DIABETES A) Laboratory findings IFG = fasting glucose levels 100 and < 126 mg/dL Glucose tolerance test between normal and diabetic (140-200 mg/dL, 2 hours after glucose load) A1C between 5.7 and 6.4%9 B) Clinical findings Increased prevalence of atherosclerosis, electrocardiographic abnormalities, hypertension, hyperlipidemia, obesity Higher age group At higher risk to develop diabetes mellitus 4. GESTATION DIABETES/IGT This class of patients is defined as women in whom during pregnancy diabetes or IGT become manifest. After pregnancy, the condition usually reverses to normal, but in some patients diabetes or IGT persists. 5. HYPERGLYCEMIA ASSOCIATED WITH CERTAIN CONDITIONS OR SYNDROMES Postpancreatectomy Cancer of pancreas Pancreatic cysts Hemorrhagic pancreatitis Acromegaly Cushing’s syndrome Pheochromocytoma Glucagonoma Somatostatinoma Primary aldosteronism Hyperthyroidism Hemochromatosis Malnutrition Many drugs and chemical agents (e.g., thyroxine, phenytoin) Over 25 rare genetic syndromes (e.g., Down’s syndrome, Klinefelter’s syndrome etc.) 9 Diagnosis and Classification of Diabetes Mellitus, Diabetes Care: 2010; 33: 562-569. 95 E. TESTS IN THE DIAGNOSIS, CLASSIFICATION AND MANAGEMENT OF DIABETES MELLITUS 1. DETERMINATION OF BLOOD GLUCOSE: WHERE, HOW AND WHEN Glucose concentration is uniform in the water phase of plasma and erythrocytes. Since, however plasma contains per unit volume 27% more water than erythrocytes, glucose levels are higher in a given volume of plasma than in an identical volume occupied by erythrocytes. For this reason plasma glucose values are higher than whole blood glucose values. Example: If glucose concentration is 120 mg/dL of water then: Erythrocytes Whole blood (Hct 45%) Plasma Water (%) 73 83 Glucose (mg/dL) 88 101 93 112 In the fasting state, glucose levels in arterial and venous blood are similar. However postprandially, arterial and capillary blood have glucose levels about 20 mg/dL higher than venous blood. This is because of the extraction of glucose by the tissues in the presence of insulin. In whole blood, clotted and kept at room temperature, glucose disappears at a rate of approximately 7% per hour owing to the ongoing glycolytic activity of leukocytes and red cells. It is thus preferable to collect blood in tubes containing fluoride, a strong inhibitor of glycolysis as well as citrate (acidity) to immediately inhibit glycolysis, or to separate the serum immediately10. The grey-stoppered Vacutainer tubes contain sodium fluoride in addition to potassium oxalate, the latter added to prevent clotting. Blood samples collected in these tubes cannot, however, be used for most other chemical examinations, particularly electrolytes, since the concentrations of sodium and potassium in this anticoagulant are: sodium = 85 meq/L, and potassium = 30 meq/L. Fluoride has a weaker inhibitory effect on the glycolysis by white cells; consequently, a significant decrease of glucose may be seen in fluoridated blood upon standing when white counts are abnormally high (50,000/L or greater). Normal and abnormal values cited below reflect measurements of glucose performed in venous plasma. We shall become familiar with these standardized values, keeping in mind the rationale and factors for conversion between plasma and whole blood glucose, and between venous and capillary glucose (in the postprandial state). 10 Gambino R et al., Acidification of blood is superior to sodium fluoride alone as an inhibitor of glycolysis, Clin Chem 55:1019-1021, 2009 96 Measurements of plasma glucose, in order to be easily interpretable, should be obtained under selected circumstances: (1) in the fasting state, or (2) during an oral glucose tolerance test. A) Fasting plasma glucose (FPG) The measurement of fasting glucose yields information on the capability of basal levels of insulin to control glycogenolysis and gluconeogenesis. When basal production (or action) of insulin is insufficient, fasting hyperglycemia develops. 1) Patient preparation The patient is kept on an average usual diet and, after an overnight fast (no food or sweetened drinks after midnight), blood is drawn in the morning. 2) Interpretation Range < 100 mg/dL 100 < 126 mg/dL ≥ 126 mg/dL Interpretation Normal May be indicative of IGT (perform glucose tolerance test) On more than one occasion is indicative of diabetes mellitus; there is no need to perform a glucose tolerance test B) Oral glucose tolerance test (OGTT)11 This test is intended to measure the capability and timely response of the insulinsecreting cells to the integrated signals provided by GI hormones and rising blood glucose levels. Although 75 g of pure glucose obviously does not constitute a typical balanced meal, the test is designed to achieve maximal sensitivity for diagnostic and epidemiologic purposes. 1) Patient preparation Put the patient for 3 days or more on a normal diet including at least 150 g of carbohydrates per day. In the morning after an overnight fast, 75 g of an aqueous solution of glucose is given. The various commercially prepared cola-flavored carbonated test drinks are convenient to use and are more palatable than a pure glucose solution. The patient should drink the solution within 5 minutes. If a patient vomits after the test drink, the test is invalid and must be repeated. Blood samples are drawn at 0 and 120 minutes. In pregnant women, i.e. for the detection of gestational diabetes, the test dose is 75 or 100 g glucose. 11 World Health Organization, Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation, Geneva: WHO Dept. of Noncommunicable Disease Surveillance, Publication No. WHO/NCD/NCS 99.2, 1999 97 In patients with carbohydrate malabsorption, an intravenous glucose tolerance test replaces the oral glucose tolerance test. Reference ranges differ. 2) Interpretation NL IGT DM F Gluc < 100 100-126 ≥ 126 2 h OGGT < 140 140-200 ≥ 200 The criteria for diagnosis of diabetes during pregnancy (gestational diabetes) are stricter than outlined above for non-pregnant adults. This is because even mild diabetes during pregnancy becomes a significant risk factor for fetal morbidity and mortality. Thus, the OGTT is performed with 75 or 100 g of glucose. The 100 g OGTT indicates gestational diabetes when two or more of the following values in mg/dL are reached or exceeded: fasting 95, 1 hour 180, 2 hours 155, 3 hours 140. The 75 g OGGT indicates gestational diabetes when two or more of the following values in mg/dL are reached or exceeded: fasting 95, 1 hour 180, 2 hour 155. 2. DETERMINATION OF KETONE BODIES Ketone bodies (acetoacetate, -hydroxybutyrate and acetone) are produced by the liver from oxidation of free fatty acids (FFA). Both release of FFA from adipose tissue stores (lipolysis) and their metabolism to ketones are highly accelerated by insulin deficiency. Thus, in the insulin-deficient state (characteristic of untreated type 1 diabetes) the liver produces ketone bodies and hydrogen ions resulting in ketoacidosis. This is often the presenting picture for juveniles who have developed type 1 diabetes. It will recur if insulin treatment is discontinued or if it is not increased when there is increased insulin demand (stress, infections, pregnancy). Ketoacidosis is rarely the presenting picture for type 2 diabetic patients; however, such patients may develop some degree of ketoacidosis under stressful circumstances. The most convenient method for estimating the degree of ketonemia and ketonuria utilizes nitroprusside tablets (Acetest) or reagent strips (Ketostix). The test is performed by reacting 0.5 mL of serum or urine with a crushed Acetest tablet, or a Ketostix. If the reaction is negative, ketoacidosis is (with the caveat discussed below) excluded. If, however, the reaction is positive (purple color development), then serial dilution of the specimen is performed until the highest dilution is established that still gives a positive reaction. Nitroprusside reacts with acetoacetate and, to a much lesser degree, with acetone (only 1/20 of the reactivity of acetoacetate), and fails to react with -hydroxybutyrate. These differences in reactivity pose a potential source of problems: during mild ketosis (fasting, suboptimal insulin effect), the ratio of -hydroxybutyrate to acetoacetate is ~2:1 and the latter ketone body is well represented, whereas in situations of prolonged, severe insulin deficiency and poor tissue oxygenation, -hydroxybutyrate will be the prevalent or exclusive ketone body present. The nitroprusside reaction may thus be only weakly positive, or negative, in spite of the presence of dangerous quantities of - 98 hydroxybutyrate. The suspicion that this is so, however, is generally high because the concomitant hyperglycemia and the calculation of the anion gap will reflect excess anions requiring identification. The finding of a level of lactic acid increased enough to indicate compromised tissue oxygenation but not sufficiently increased to account for the anion gap suggests the presence of ketonemia due to -hydroxybutyrate. It is interesting that, in such cases, the positivity of the nitroprusside test for serum ketones will become stronger as treatment with insulin and fluid is improving the patient’s status. 3. DETERMINATION OF INSULIN AND C-PEPTIDE Insulin is synthesized first as a precursor molecule, proinsulin. The A and B chains in proinsulin are held together by a connecting peptide, called C-peptide. Proinsulin is then converted in the beta cells to insulin which is secreted together with C-`ide in equimolar amounts. However, the t1/2 of C-peptide is longer than that of insulin. C-peptide assays do not react with insulin antibodies. Insulin has been synthesized commercially for clinical use by recombinant DNA technology and is now available in this form. Neither determination of insulin or C-peptide is generally warranted for initial diagnosis of diabetes. They are mostly used to verify classification and for various investigational purposes. The availability of immunoassays for C-peptide has been extremely useful in permitting studies of insulin secretory capacity of the pancreatic -cells of patients on various drug therapies. 4. GLYCOSURIA Glycosuria appears when the blood glucose level exceeds the renal threshold for reabsorption of glucose. This normally lies at about 170 mg/dL, but it may be lower in renal tubular disease or elevated in diabetics to above 300 mg/dL. Thus glycosuria may be absent in these patients despite markedly elevated blood glucose levels. Non-diabetic causes of glycosuria are: 1) Glycosuria not associated with hyperglycemia Pregnancy Fanconi’s syndrome Renal glycosuria (“true renal diabetes”) Nephrotic syndrome 2) Glycosuria associated with hyperglycemia Thyrotoxicosis Cushing’s syndrome Damage to CNS Infections Anesthesia Excitement and stress 99 Semi-quantitative tests for glycosuria are described under routine urinalysis. They are based either on the use of glucose oxidase as in the dipstick or on copper reduction methods as in the tablet test (Clinitest). Quantitative urinary glucose determinations are seldom necessary. The glucose concentrations in the urine of diabetics are commonly in the range of 0.5 to 2.0 g/dL, but values over 5 g/dL are occasionally encountered. The effect of high glucose concentration on urinary specific gravity should be kept in mind. 5. GLYCATED-HEMOGLOBIN During the 120 day life span of the red cell, hemoglobin A (and other forms) becomes glycated due to the non-enzymatic, largely irreversible, post-translational attachment of a molecule of glucose to the terminal valine of the -globin chain and to -amino groups of lysines of both - and -chains. The degree of glycation is directly proportional to the level of glucose in the blood, and it has been shown that the amount of glycated hemoglobin present in blood is a reflection of the average blood glucose level over the life span of a red cell. Thus, the quantitative determination of glycated hemoglobin has become a useful adjunct in the assessment of the efficacy of long-term therapeutic control of diabetic patients. Glycated hemoglobin results are reported as % HbA1c. HbA1c by ion exchange At UCSD and VA Medical Centers, the analysis of Hemoglobin A1c is performed by high performance liquid chromatography (HPLC) with a cation exchanger. Reference Range: 3.8-6.3%; Target for therapy is < 7%12 Hemoglobin variants can affect the measurement of A1c by ion exchange. The most common hemoglobin variants, AS and AC trait do not effect the measurement of A1c by the methods currently used by the VA and UCSD hospitals, but the effect of other variants is unknown. When a patient has a hemoglobin variant other than AS or AC trait, hemoglobin A1c reference ranges may not apply. In these cases A1c should only be used to monitor therapy after obtaining a baseline value. If t1/2 of red cells is shorter, as in some hemoglobinopathies the HbA1c is underestimated. Abnormal red cell survival will also affect interpretation of hemoglobin A1c. Samples from patients with hemolytic anemia will have lower A1c values due to the decreased lifetime of the red blood cells. Samples from patients post-splenectomy and patients with polycythemia may exhibit increased A1c values due to the longer half-life of red blood cells in these patients. Hemoglobin variants that effect red blood cell survival will also effect interpretation of A1c. 12 Skyler JS et al., Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association, Diabetes Care 32:187-192, 2009 100 One formula for estimating average glucose (AG) based on HbA1c values in stable diabetic patients is13: AG = 28.7 (HbA1c) - 46.7 6. GLYCATED PROTEINS Due to their shorter plasma half lives, glycated proteins such as glycated albumin may be useful in assessing the average blood glucose concentration over one to two weeks. The most popular method at this time is the fructosamine assay which is a measure of glycated plasma proteins (primarily Albumin). Since the t1/2 of Hb is 60 days, HbA1c values reflect glycemic control for the previous 6-8 weeks. For fructosamine, the period of glycemic control is based on albumin (t1/2 = 18 days) and a variety of other proteins (t1/2 range from 2.5 to 23 days). Therefore, fructosamine reflects control over the previous 2-3 weeks. While this method is still being critically evaluated it is being used at some sites. It should be used with caution as many investigators believe that the assay is not reliable. 7. PROTEINURIA Used to monitor progress of renal involvement of diabetes Angiotensin-converting enzyme inhibitors (ACE) appear to be beneficial in preventing further loss of function Diabetic patients, even with recent onset, have increased urinary excretion and clearance of proteins with a MW higher than 40,000 when compared with non-diabetics. This increased glomerular permeability can become non-selective for the larger proteins and may progress to several grams of protein loss/24 hour. Since the appearance of proteinuria (albumin) is the earliest sign of renal involvement by the diabetic process, it is advisable to perform at least semi-quantitative tests for proteinuria eventually to be followed by precise quantitation (see Urinalysis) for albumin. The first signs of renal damage are signified by the appearance of microalbuminuria. (Microalbuminuria is a misnomer, which implies small amounts of albumin and not a small, abnormal molecule of albumin). Microalbuminuria may occur at a time when there is not yet any evidence of decreased glomerular filtration rate or evidence of glomerular lesions; it is a strong, early predictor of impending nephropathy in diabetic patients. Microalbuminuria is not detected with routine protein methods, and requires special techniques. The preferred way to measure proteinuria includes normalizing the value to the amount of creatinine excreted and reporting an albumin to creatinine ratio. 8. ANTI-INSULIN ANTIBODIES Before the availability of highly purified animal insulin preparations and recombinant human insulin, development of IgG antibodies to insulin could be demonstrated in nearly all patients receiving insulin therapy for more than a few weeks. With the currently 13 Nathan DM, et al., Translating the A1C assay into estimated average glucose values, Diabetes Care 31:14731478, 2008 101 increasing use of human insulin preparations problems of immunologic resistance to insulin will hopefully decline and disappear. Table 2: Laboratory Tests in the Diagnosis and Management of Hyperglycemic Emergencies Plasma glucose SUN Creatinine Ketone bodies Osmolality Sodium Potassium Bicarbonate Chloride Blood pH Table 3: Criteria for the Diagnosis of Diabetes14 1. A1c ≥ 6.5%. The test should be performed in a laboratory using a method that is NGSP-certified and standardized to the DCCT assay.* OR 2. FPG ≥ 126 mg/dl (7.9 mmol/L). Fasting is defined as no caloric intake for at least 8 h.* OR 3. Two-hour plasma glucose ≥ 200 mg/dl (11.1 mmol/L) during an OGTT. The test should be performed as described by the World Health Organization, using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water.* OR 4. In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 200 mg/dl (11.1 mmol/L). * 14 In the absence of unequivocal hyperglycemia, criteria 1-3 should be confirmed by repeat testing. American Diabetes Association, Standards of Medical Care in Diabetes—2010, Diabetes Care 33:S11-S61, 2010 102 F. CASE STUDIES CASE 1 (Diabetes Technol. Ther. 4(4):505-514, 2002) The patient is a 54-year-old postmenopausal, morbidly obese Hispanic woman with a history of asthma, allergic rhinitis, hypertension, and type 2 diabetes for 10 years (baseline A1c 7.1). Noncompliance with diet and exercise resulted in a 10 lb (4.5 kg) weight gain within an 8month period. The patient also started to exhibit poor glycemic control, resulting in HbA1c values of 6.7%, 7.9%, and 7.5% over 13 months. The patient adamantly refused insulin therapy; therefore, a trial of rosiglitazone 4 mg once a day was added to the existing combination therapy of extended-release glipizide 20 mg once daily and metformin 1 g BID. After 6 weeks of therapy with rosiglitazone, the dose was increased to 8 mg/day (given in two divided doses per day) to achieve better glycemic control as exhibited by an HbA1c of 6.5% at 3 months. Within 3 months of the dose escalation, the patient began complaining of bilateral pedal edema below the knee all the way to the feet, accompanied by moderate pain upon walking or standing but no pain at rest. The dose of rosiglitazone was reduced to 4 mg, and diuresis therapy was not initiated. At the 2-month follow-up, bilateral pitting edema remained evident, and rosiglitazone was discontinued. An overall 11 lb (5 kg) weight gain was recorded for the 8-month period of rosiglitazone treatment. Rosiglitazone was discontinued for a 6-month period and only trace amounts of bilateral pedal edema remained. However, this patient’s glycemic control continued to worsen with an ensuing HbA1c of 7.9%. 1. Based on this patient’s baseline HbA1c of 7.1% calculate her mean blood glucose before receiving drug therapy. 2. What was her mean blood glucose when her HbA1c was 6.5%? 3. What are possible drug-induced explanations for her edema? Is edema a common ADR for this class of drugs? 4. Since this patient is failing drug therapy what alternative drug therapies would you suggest? 103 CASE 2 A 10-year-old boy, in excellent health until 1 month ago, is noted to have become irritable in school and at home and somewhat lethargic after meals. Because his 15 year old brother has recently developed diabetes, the mother is concerned and asks the pediatrician to evaluate the boy for diabetes. Fasting plasma glucose on two occasions is 130 and 140 mg/dL. A random plasma glucose in the afternoon is 190 mg/dL, HbA1c is 8.5%, and there is no ketonuria. A nearby laboratory is requested to analyze a serum specimen for islet-cell antibodies, which are found to be present at a titer of 1:4. 1. Should the boy be considered to have diabetes, and (if so) what type? 2. Should one perform further testing and (if so) what tests should be ordered? CASE 3 A 68-year-old obese woman is brought semi-comatose to the emergency room. She has a history of seizures and hypertension and has been treated with phenytoin and thiazide diuretics. She is not known to have diabetes. On physical examination she exhibits a right hemiparesis. Initial laboratory results: plasma glucose 1,080 mg/dL, serum sodium 144, potassium 4.4, chloride 113, bicarbonate 20 mmol/L, SUN 60 mg/dL. 1. Calculate the osmolality. 2. Is the serum sodium appropriate for the degree of hyperglycemia? 3. What is the effect of the hyperosmolality on the hematocrit? 4. Do you expect ketoacidosis to be present? 5. Why are the SUN and chloride increased, and the bicarbonate decreased? 104 IX ELECTROLYTES AND ACID-BASE BALANCE 105 106 A. OBJECTIVES To describe the most important hormones related to electrolyte and acid-base balance To be able to calculate anion and osmol gaps To understand the major clinical causes for electrolyte and acid-base disturbances To be able to interpret blood gas data in terms of acid-base status To be able to differentiate acidosis from alkalosis and to determine if the primary cause is respiratory or metabolic B. KEY TERMS Acidosis - arterial blood pH < 7.35 Alkalosis - arterial blood pH > 7.45 Anion gap - the difference between the measured cations and measured anions (Na+ + K+) (Cl- + HCO-3) Metabolic alkalosis - primary excess of bicarbonate Metabolic acidosis - primary deficit of bicarbonate Osmol gap - the difference between the measured osmolality and the calculated osmolality Osmolality - the number of particles dissolved in a kg of water Respiratory acidosis - primary excess of dCO2 Respiratory alkalosis - primary deficit of dCO2 C. BACKGROUND/SIGNIFICANCE A basic understanding of renal and lung physiology is assumed for this chapter. Electrolyte analyses are among the most frequently ordered laboratory tests and an understanding acidbase balance is important to every clinician. Many drugs have a direct effect on acid-base balance while others influence pH through pharmacological mechanisms. Understanding how to interpret blood gas and electrolytes forms the core for the diagnosis and treatment of a variety of pathological states. 107 D. SAMPLE COLLECTION FOR ELECTROLYTE ANALYSIS Plasma: Ammonium heparinate is the preferred anticoagulant. Do not use EDTA, citrate, oxalate or fluoride anticoagulants since these may elevate sodium or potassium if these salts are used; also, some bind Ca++ and Mg++. Serum: Potassium values are slightly higher in serum (0.1 - 0.6 mmol/L) than in plasma because of release of potassium from platelets during clotting. Consequently spuriously high potassium values may be seen in severe thrombocythemia (3 million/L). In this condition, plasma potassium rather than serum potassium should be used. Long storage of whole blood specimens results in release of potassium from leukocytes and erythrocytes. Refrigeration of the clotted sample will result in elevation of serum potassium and depression of serum sodium due to inhibition of the Na/K-ATPase pump. This process does not produce hemolysis. HEMOLYSIS AND PLASMA POTASSIUM VALUES For each 30 mg/dL increase of plasma hemoglobin, potassium rises 0.1 mmol/L due to release of K+ from erythrocytes 30 mg of hemoglobin/dL of plasma = barely pink 60 mg of hemoglobin/dL of plasma = red 90 mg of hemoglobin/dL of plasma = burgundy E. HORMONES IMPORTANT IN ELECTROLYTE AND ACID-BASE BALANCE 1. ADH (VASOPRESSIN) Produced in hypothalamus, stored in pituitary Causes water to be reabsorbed by collecting ducts Release regulated by osmolality Diabetes insipidus - kidneys do not respond to ADH (or no ADH produced) 2. ALDOSTERONE 108 Promotes retention of Na+ and HCO-3 and excretion of K+ and H+ with retention of water Promotes retention of Na+ and Cl- from sweat gland Released by angiotensin II (renin released due to decreased renal perfusion and hyponatremia) Addison’s disease- destruction of adrenal cortex leading to deficit of aldosterone and cortisol Conn’s syndrome-hyperaldosteronism 153 mEq/L mEqmEq/ Cation s 153 mEq/L Anion s 10 3 Cl - 14 2 Na + Inorganic acids 2 Organic acids 5 Proteins Unmeasured 4 5 2 K+ Ca ++ HCO 3- Mg ++ Unmeasured 1 6 2 7 Figure 1: “Gamble-Gram” shows that the Intravascular Concentrations of Cations are balanced by Anions F. THE “ANION GAP” In clinical practice, only sodium, potassium, chloride, and bicarbonate are routinely measured in order to evaluate the electrolyte balance in the patient. This leaves approximately 7 mmol/L of “undetermined cations” (calcium, magnesium), as well as approximately 23 mmol/L of “undetermined anions” (phosphate, sulfate, organic acids, proteins - see “Gamble-Gram”). When only the cations sodium and potassium and the anions chloride and bicarbonate are balanced against each other, a difference in unmeasured ions, the so-called “anion gap” results, which normally ranges from 10-20 mmol/L (average ~16). “Gap” is a misnomer since there is always a balance. The anion gap is simply a reflection of the difference in the number of “measured cations” and “measured anions” and therefore also of “unmeasured cations” and “unmeasured anions.” (Na+ + K+) - (Cl- + HCO-3) = 16 (range: 10-20) Using the values of the illustration (Gamble-Gram): (142 + 4) - (103 + 27) = 146 - 130 = 16 (range: 10-20) Alternative formula: (Na+) - (Cl- + HCO-3) 142 - (103 + 27) = 12 (range: 7-16) The anion gap is influenced by changes of the unmeasured ions. The most frequent change is an increase of the anion gap, indicating acidosis due to accumulation of acid metabolites (organic acids such as ketoacids, phosphate, and sulfate) as in renal disease or uncontrolled diabetes. Less frequently a decrease of the anion gap is seen, which may be due to 109 hypoproteinemia, the presence of a cationic paraprotein as in multiple myeloma, or an increase in calcium or magnesium (“undetermined cations”). Calcium, magnesium, phosphate, and protein are usually determined for reasons other than their role in electrolyte or acid-base balance. 1. MAJOR CLINICAL CAUSES OF AN INCREASED ANION GAP Ketoacidosis (diabetic, alcoholic, starvation) caused by acetoacetate and hydroxybutyrate Renal failure (accumulation of organic acids, sulfuric acid, phosphoric acid) Lactic acidosis Treatment with substances that are unmeasured anions at physiological pH, e.g. citrate, lactate, carbenicillin, penicillin Poisonings (all yield unmeasured anions) Aspirin, salicylic acid, and other organic acids Methanol (formic acid metabolite) Ethylene glycol (glycolic and oxalic acid metabolites) Paraldehyde (acetic acid metabolite) 2. MAJOR CLINICAL CAUSES OF A DECREASED ANION GAP Hypoalbuminemia (decrease in negative charge) Hemodilution Paraproteins increase unmeasured cations, due to positive charge G. SERUM OSMOLALITY Osmolality (not osmolarity) is the number of particles of solute (osmolutes) dissolved in a kilogram of water. It is measured in units of milliosmoles per kilogram of water. For a nondissociable, water-soluble compound (e.g., glucose, urea, ethanol), one “osmole” equals one mole, and one “milliosmole” equals one millimole. In the case of a dissociable, watersoluble compound the number of particles yielded per mole must be considered, e.g. 1 mole of NaCl yields theoretically 2 moles of solute. Thus: For glucose (MW 180): 1 milliosmol e L of serum water 180 mg L 18 mg dL For urea measured as urea-N (2 nitrogens per molecule of urea; AW of N =14; 2 N=28): 1 milliosmol e L of serum water 110 28 mg L 2.8 mg dL For ethanol (MW 46): 1 milliosmol e L of serum water 46 mg L 4.6 mg dL Thus serum osmolality may be calculated as follows based on measured values: 2 (Na in mmol/L) + Glucose in mg/dL Urea N in mg/dL + = osmolality 18 2.8 In healthy persons the osmolality is (assuming normal Na 140, glu 90, and SUN 14): 2 (Na) + (glu) SUN 90 14 + = 2 (140) + + = 290 mOsm/kg 18 2.8 18 2.8 1. MEASUREMENT OF OSMOLALITY This analysis is based on the measurement of the freezing-point depression of water due to solutes in solution. Freezing-point depression is linearly related to solute concentration. When one mole of any nonionic solute is dissolved in a kilogram of water, the freezing point is lowered by 1.858C. A mole of an electrolyte or ionic substance will lower the freezing point a multiple of this amount depending on how many ions are formed when the substance is dissolved. Reference Range: 270-310 mOsm/kg It must be emphasized that osmolality is usually measured on serum, not plasma, since anticoagulants such as EDTA, oxalate, fluoride, etc. contribute to osmolality (some as much as 155 mOsm/kg!). Heparinized plasma, however, can be used. 2. MAJOR CLINICAL CAUSES OF HYPEROSMOLALITY Dehydration Hyperglycemia/diabetic ketoacidosis Diabetes insipidus (serum osmolality high, but urine osmolality low) Uremia Ethanol ingestion Improper specimen collection (use of anticoagulants - this effect is enhanced in a partially filled tube) 3. MAJOR CLINICAL CAUSES OF HYPOSMOLALITY Over hydration Inappropriate antidiuretic hormone (ADH) secretion (SIADH) (serum osmolality low but urine osmolality high) Compulsive water drinking (psychogenic polydipsia) 111 4. OSMOLAL GAP The difference between measured osmolality and calculated osmolality is called the osmolal gap. A high osmolal gap suggests either the presence of another compound (e.g., ethanol) whose identity should be sought, or elevation of endogenous constituents that may not have been measured (e.g., proteins, ketoacids). H. MAJOR CLINICAL CAUSES OF ELECTROLYTE DISTURBANCES 1. SODIUM Reference values Serum: 135-145 mmol/L Erythrocytes: 16 mmol/L (thus, hemolysis lowers serum sodium by dilution) A) Hypernatremia Dehydration Diarrhea (water loss) Hyperadrenalism (Cushing’s syndrome) Aldosteronism B) Hyponatremia Over hydration Diarrhea (sodium loss) Intestinal fistula Addison’s disease (hypoadrenalism) Renal disease, tubular dysfunction Salt losing nephritis Uncontrolled diabetes (cations lost with keto-acids in urine) Dilutional hyponatremia associated with hyperglycemia (osmotic effect); for each 100 mg/dL glucose over normal, sodium is lowered 1.6 mmol/L Prolonged use of diuretics Inappropriate antidiuretic hormone secretion (SIADH) B-2) 112 Hyponatremia with increased total body sodium Renal insufficiency Congestive heart failure (water retention, edema) Hepatic cirrhosis with ascites Nephrotic syndrome Protein deficiency 2. POTASSIUM Reference values: Serum: 3.5-5.0 mmol/L Erythrocytes: 125 mmol/L (thus, hemolysis increases serum potassium) A) Hyperkalemia Tissue damage or impairment of renal clearance of K+ Shock Uncontrolled diabetes mellitus (tissue breakdown, utilization of protein for calories) Dehydration Adrenocortical insufficiency (Addison’s disease) B) Hypokalemia Poor food intake Prolonged intravenous glucose or NaCl (without K+) Vomiting GI fistulas (mostly intestinal) Diarrhea Large intestinal adenomas (mucus-producing) Aldosteronism Hyperadrenalism Over dosage with ACTH and cortisone Familial periodic paralysis (intracellular K+ high) Diuretic abuse Laxative abuse Plasma Na+ HCO3- Tubular Cell Na+ HCO3- H+ H2CO3 CO2 + H2O Glomerular Filtrate Na+ HCO3H+ H+ HCO3- H2CO3 CO2 + H2O Figure 2: Renal Reclamation of Bicarbonate 113 THE HENDERSON-HASSELBALCH EQUATION AND THE BICARBONATE/CARBONIC ACID BUFFER SYSTEM The bicarbonate/carbonic acid buffer system is the most important buffer system in plasma for maintenance of physiological pH. The Henderson-Hasselbalch (H-H) equation may be used to appreciate the concepts of respiratory and metabolic acidosis and alkalosis: pH = pK'a + log [base] [HCO-3] [24 mmol/L] = pK'a + log = 6.1 + log [acid] [H2CO3] [1.2 mmol/L] = 6.1 + log 20 = 6.1 + 1.3 = 7.4 - H CO H O CO (Pulmonary ) Also recall that : ( Renal ) H HCO3 2 3 2 2 The H - H equation indicates that the ratio of HCO-3/ H2CO3 (which is normally 20/1) determines the blood pH. Any change, whether in the numerator (HCO-3) or in the denominator (H2CO3), will cause a change in pH. In metabolic acidosis, the H+ reacts with the bicarbonate component of the buffer system to form carbonic acid, which forms CO2 + H2O. The CO2 is removed by increased ventilation. In this reaction, HCO-3is lost (= bicarbonate deficit). H+ + HCO-3 → H2CO3 → CO2 ↑ + H2O (Pulmonary) In metabolic alkalosis, the OH- reacts with the carbonic acid component of the buffer system to form HCO-3 (=bicarbonate excess) OH- + H2CO3 → HCO-3 + H2O 3. BICARBONATE Reference values: 22-28 mmol/L Newborns: 19-23 mmol/L, adult level reached by 2-6 months A) Increased plasma bicarbonate 1) Respiratory acidosis (primary imbalance is dCO2 excess) a) Causes Prolonged hypoventilation (CO2 retention, increased H2CO3, decreased pH) Central nervous system depression (e.g., opiate usage) Pulmonary disease (e.g., emphysema, fibrosis, pulmonary obstruction) Cardiac disease 114 b) Compensatory mechanisms Hemoglobin and protein buffer systems Hyperventilation Na+ - H+ exchange H2PO4- formation HCO-3 reclamation NH3 production c) Laboratory findings Plasma pCO2 , pH After compensation HCO-3 , tCO2 Urine pH 2) Metabolic alkalosis (primary imbalance is HCO-3 excess) a) Causes Loss of acid from stomach (prolonged vomiting) Excessive drainage of stomach (iatrogenic) Cushing’s syndrome (hyperadrenalism), loss of KCl Excessive ACTH or cortisone (KCl is lost in excess in urine) Use of diuretics b) Compensatory mechanisms HCO-3 /H2CO3 buffer system Hypoventilation Na+ - H+ exchange NH3 production HCO-3 reclamation c) Laboratory findings HCO-3 , tCO2 ; pH After compensation: pCO2 ; pH 115 Plasma Tubular Cell Glomerular Filtrate CO2 + H2O HCO3Na+ HCO3- H+ H+ Na+ Na+Na+HPO4 H+A- glutamine NH4+ glutamate NH3 NH4+ NH3 -ketoglutarate NaH2PO4 NH4+A- urine Figure 3: Renal Excretion of Acid, Sodium/Hydrogen Ion Exchange and Formation of Ammonia B. Decreased plasma bicarbonate 1) Respiratory alkalosis (primary imbalance is decrease in dCO2) a) Cause Increased rate and depth of respiration (fever, high external temperatures, hysteria, anoxia; an early phase of salicylate poisoning) b) Compensatory mechanisms NH3 production Na+ - H+ exchange HCO-3 reclamation c) Laboratory findings pCO2 , pH After compensation: HCO-3 , tCO2 , pH 2) Metabolic acidosis (primary imbalance is decrease in HCO-3) a) Causes Overproduction of acids (diabetes, lactic acidosis) Reduced H+ excretion (renal failure, renal tubular acidosis, decreased Na+ - H+ exchange) Excessive loss of base (diarrhea, GI fistula) 116 b) Compensatory mechanisms HCO-3 / H2CO3 buffer system Hyperventilation Na+ - H+ exchange H2PO4- formation NH3 production HCO-3 reclamation c) Laboratory findings HCO-3 , tCO2 , pH After compensation: pCO2 , pH 4. CHLORIDE Reference values: Serum: 97-107 mmol/L Erythrocytes 52 mmol/L (thus, hemolysis lowers serum chloride) A) Hyperchloremia Dehydration Hyperchloremic acidosis (loss of HCO-3 due to diarrhea or renal tubular acidosis) Stimulation of respiratory center (drugs, hysteria, anxiety, fever, hyperventilation) causes loss of CO2 and decrease in HCO-3 High altitudes (small effect which is due to hyperventilation) B) Hypochloremia Overhydration Hypoventilation (CO2 retention) Depression of central nervous system (CO2 retention) Pulmonary disease Chronic renal disease Diabetic ketosis Adrenal insufficiency (Cl- lost from kidney together with Na+) Hyperfunction of adrenal cortex (Cl- lost from kidney with K+) Over dosage with ACTH and cortisone (hypochloremic alkalosis) Metabolic alkalosis Vomiting Fistulas of GI tract (gastric) Note: The compensatory increase/decrease in chloride “offsets” the corresponding changes in bicarbonate, thereby preserving electroneutrality. 117 I. OTHER CLINICALLY IMPORTANT IONS 1. CALCIUM 5th most common element in body Average human has 1 kg of Ca Three compartments, skeletal (99% of Ca), soft tissue, and extracellular Three forms, free Ca++ (50%, ionized), protein bound (40%), complexed to small anions (10%) Ionized calcium is regulated by 1,25-dihydroxyvitamin D, parathyroid hormone (PTH) and calcitonin Reference range 8.5-10.4 mg/dL Interpretation of Calcium Since extensively bound to albumin, need to consider albumin concentrations when interpreting Ca++ Monitored for a variety of reasons including: bone disease, malignancy, hyperand hypoparathyroidism, renal disease, and endocrine disorders 2. PHOSPHATE Adults have 600 g (85% in bone) Reference range 2.5-4.5 mg/dL Critical role in high energy compounds (e.g., ATP, NADP) Essential element in phospholipids Phosphorylation controls a variety of enzymatic and nuclear transcription factors 3. MAGNESIUM Total body content of about 25 g (55% in skeleton) Reference range 1.7-2.6 mg/dL A co-factor for over 300 enzymes Estimated that 10% of patients admitted to hospitals have low magnesium Hypokalemia commonly present with low magnesium J. HORMONES IMPORTANT FOR REGULATING BONE AND MINERAL METABOLISM 1. PARATHYROID HORMONE 118 Synthesized and secreted by parathyroid glands Increases serum Ca by increasing bone resorption Decreases serum phosphate by increasing excretion Increases 1,25-dihydroxyvitamin D3 Release regulated by ionized Ca++ Important to meaure Ca++ and PTA at same time 2. 1,25-DIHYDROXYVITAMIN D3 Dietary vitamin D3 is sequentially hydroxylated in liver to form 25-hydroxyvitamin D3 and then in the kidney to form the active hormone 1,25-dihydroxyvitamin D3 Increases Ca++ and phosphate absorption in gut With PTH increases osteoclast activity Increases kidney reabsorption of Ca++ and phosphate Thought to be important in variety of chronic diseases More than 50% of adults have insufficiency 3. CALCITONIN Secreted by parafollicular cells of thyroid gland Reduces concentrations of Ca++ and phosphate Inhibits osteoclastic bone reabsorption Exact physiological role is uncertain 119 K. CASE STUDIES CASE 1 A febrile 84-year-old woman is brought from a nursing home to the emergency department of a local hospital. She is severely cachectic, confused, has sagging skin folds, and extremely dry skin. Admission chemistry tests are: serum sodium 168 mmol/L, serum potassium 6.2 mmol/L, chloride 130 mmol/L, and HCO-3 26 mmol/L. The serum osmolality is found to be 356 mOsm/kg of water. A urea nitrogen drawn at admission is 38 mg/dL and the patient’s hematocrit is 58%. 1. Explain the elevated serum sodium and the elevated serum osmolality. 2. Explain the elevated urea-N. 3. What other laboratory studies are indicated? 4. Explain the high hematocrit in this patient. 5. Propose possible reasons for this patient’s abnormal laboratory data. 120 CASE 2 A 40-year-old male presents to the emergency department confused and with the odor of alcohol on his breath. Chemistry tests performed at the time of admission show the following: serum sodium 137, potassium 4.2, chloride 100, and HCO-3 26 mmol/L. The ureaN was 14 mg/dL and glucose was 90 mg/dL. The serum osmolality, however, is 360 mOsm/kg of water. 1. Propose a reason for the patient’s elevated serum osmolality. 2. How would you test your hypothesis? 3. Discuss reasons for serum hyperosmolality in the emergency department patient. 4. Calculate the osmolality based on the above data. 5. Assuming the elevated osmolality is due to ethanol, calculate the patient’s expected blood ethanol concentration. 121 CASE 3 An 8-year-old girl was brought to the hospital with a 4-day history of profuse diarrhea. She was listless and responded rather incoherently to questions. Her skin turgor was poor for a child her age, and her eyes were soft and sunken. Pulse was 114 beats/minute with a BP of 98/66 mmHg. Respirations were deep and at a rate of 26/minute. Hematocrit was 58%. Lungs were clear, and the abdomen was soft without evidence of significant local tenderness. The following laboratory data were obtained: pH pO2 Na+ Serum Electrolytes: ClABG: = 7.13 = 96 mmHg = 133 mmol/L = 115 mmol/L pCO2 HCO-3 K+ total CO2 = 18 mmHg = 6 mmol/L = 3.1 mmol/L = 7 mmol/L 1. What is the nature and etiology of the acid-base disorder in this patient? 2. Is there evidence for and expected degree of compensation for this disorder? 3. Explain the low serum potassium. 4. Is there evidence for abnormalities of electrolyte fluid balance? If so, how might such a disturbance impact serum potassium? 5. Is serum K+ a good indicator of total body K+ for patients with acid-base abnormalities? 6. What are the causes of the high pulse rate, low blood pressure, and high respiratory rate? 7. Why is the serum chloride increased? 122 CASE 4 A 21-year-old woman with an eight-year history of juvenile onset diabetes was brought to the hospital in a coma. She had required 92 units of insulin daily to maintain her blood glucose concentration in an acceptable range and prevent excessive glucosuria. On admission she had a BP of 92/20 mmHg, a pulse of 122 beats/min, and deep respirations of 32/min. Lab data showed: pH pO2 Na+ Serum ClChemistry glucose Values: creatinine ABG: = 7.10 = 90 mmHg = 129 mmol/L = 95 mmol/L = 1200 mg/dL = 2.3 mg/dL pCO2 HCO-3 K+ total CO2 urea nitrogen = 15 mmHg = 4 mmol/L = 6.4 mmol/L = 5 mmol/L = 74 mg/dL The serum was strongly positive for ketones. Eight units of regular insulin were given IV and 8 units/h were given by IV infusion pump. Her serum glucose concentration fell at a rate of approximately 100 mg/dL each hour. In seven hours her ventilation and blood pH were normal following IV injection of NaHCO3 and vigorous fluid and electrolyte replacement. 1. What is the nature and etiology of the acid-base disturbance? 2. Is there indication for a normal compensatory response? 3. What are serum ketones (ketone bodies)? How are they frequently detected? 4. Explain the abnormal serum potassium result. 5. Explain the low serum sodium result. 6. What is the cause of the low BP upon admission? How does the low BP affect GFR (glomerular filtration rate)? 7. Calculate the patient’s anion gap. Explain. 8. Calculate the patient’s osmolality. Interpret. 9. What is the patient’s corrected sodium. Interpret. 123 CASE 5 (Nephrol. Dial. Transplant. 14(1):226-230, 1999) A 54-year-old male was admitted to emergency department with progressive weakness, somnolence, and shortness of breath 5 days after receiving chemotherapy (ifosfamide 2 g/m2) for recurrence of sarcoma. Past medical history included diabetes mellitus type II that was diet controlled. Initial labs: creatinine 3.5 mg/dL, K+ 2.3 mmol/L, Na+ 147 mmol/L, Cl- 122 mmol/L, glucose 400 mg/dL. Initial therapy included isotonic saline, 60 mmol of KCl and subcutaneous insulin. On day 2 similar labs as above were obtained. Blood gases were measured and an initial diagnosis of ketoacidosis was made. Patient was admitted to ICU with blood pressure of 120/60 mmHg and heart rate regular at 120/min. Respiration rate was 36/min, deep, and labored. Labs: Analyte Na+ K+ ClGlucose Creatinine pH pCO2 pO2 HCO-3 Units Day 3 Day 4 Reference Range mmol/L 160 162 135 - 145 mmol/L 2.3 4.0 3.5 - 5.2 mmol/L 140 132 97 - 108 mg/dL 300 307 76 - 110 mg/dL 3.7 4.4 0.3 - 1.2 7.24 7.46 7.34 - 7.44 mmHg 12 20 35 - 46 mmHg 122 87 69 - 116 mmol/L 5 14 22 - 26 1. The initial therapy included KCl and insulin. Why was the KCl necessary? 2. Were any important lab results missing from the initial studies? 3. How do you interpret the creatinine values? 4. Calculate the anion gap for day 3. Does this support the initial diagnosis of ketoacidosis? 5. What is the nature of this acid-base disturbance? 6. Is there evidence of compensatory mechanisms? 7. Propose a drug-induced mechanism for these laboratory results. Is this adverse reaction typical of this class of drugs? 124 Table 1: Classification and Characteristics of Simple Acid-Base Disorders Primary Change Compensatory Response Expected Compensation METABOLIC Acidosis cHCO-3 pCO2 Alkalosis cHCO-3 pCO2 Acute pCO2 cHCO-3 Chronic pCO2 cHCO-3 Acute pCO2 cHCO-3 Chronic pCO2 cHCO-3 pCO2 = 1.5 (cHCO-3) + 8 2 pCO2 falls by 1-1.3 mmHg for each mmol/L fall in cHCO-3 Last 2 digits of pH = pCO2 (e.g., if pCO2 = 28, pH = 7.28) cHCO-3 + 15 = last 2 digits of pH cHCO-3 = 15, pH = 7.30 pCO2 increases 6 mmHg for each 10 mmol/L rise in cHCO-3 cHCO-3 + 15 = last 2 digits of pH (cHCO-3 = 35, pH = 7.50) RESPIRATORY Acidosis cHCO-3 increases by 1 mmol/L for each 10 mmHg rise in pCO2 cHCO-3 increases by 3.5 mmol/L for each 10 mmHg rise in pCO2 Alkalosis cHCO-3 falls by 2 mmol/L for each 10 mmHg fall in pCO2 cHCO-3 falls by 5 mmol/L for each 10 mmHg fall in pCO2 Modified from Narins RG and Gardner LB, Simple acid-base disturbances, Med. Clin. North Am. 65:321-346, 1981 [Tietz Textbook of Clinical Chemistry, 3rd edition, table 32-4, p. 1115]. 125 Figure 4: Acid-Base Map: area of normal values is labeled N; map actually extends further up than shown (to a pH of 6;6) and further to right than shown (to a pCO2 of 180 mmHg); numbered lines represent isopleths for bicarbonate (in milliequivalents per liter); from Goldberg M et al., Computerbased instruction and diagnosis of acid-base disorders: a systematic approach, JAMA 223:269-275, 1973, p. 270 126 Figure 5: The Siggaard-Anderson Alignment Nomogram for the Calculation of Acid-Base Parameters 127 128 X LABORATORY DIAGNOSIS OF ADRENOCORTICAL DISEASE 129 130 A. OBJECTIVES To describe the relationships between hormones and steroids in the hypothalamus, pituitary and adrenal gland To describe the laboratory tests used to assess hyper and hypocortisolism To describe the laboratory diagnosis of Cushing’s syndrome, Addison’s disease, Conn’s syndrome and congenital adrenal hyperplasia B. KEY TERMS Adrenocorticotrophic hormone (ACTH) - secreted by the anterior pituitary, acts primarily on the adrenal cortex to increase growth and secretion of corticosteroids Addison’s disease - destruction or dysfunction of the adrenal glands, primary adrenal insufficiency Congenital adrenal hyperplasia - absence of one or more of the enzymes needed for cortisol synthesis leading to compensatory increase in ACTH and hyperplasia of adrenals Conn’s syndrome - primary hyperaldosteronism Cortisol - major adrenal glucocorticoid which causes increased gluconeogenesis, protein catabolism, and lipid breakdown; also sensitizes arterioles to norepinephrine and has anti inflammatory and immunosuppressive effects Corticotrophin releasing hormone (CRH) - secreted by hypothalamus, causes release of ACTH from anterior pituitary Cushing’s syndrome - Result of excessive cortisol C. BACKGROUND/SIGNIFICANCE For the proper understanding of the principles on which the diagnostic tests of adrenocortical function are based, it is desirable to understand the histology, biochemistry, physiology and pathology of the pituitary and adrenal glands and their functional interrelationships. Figure 3 in this chapter shows the interrelationship of the hypothalamus, pituitary and adrenal gland. The diagnostic approach to the following conditions is discussed below: Hypercortisolism Hypocortisolism Congenital adrenal hyperplasia Hyperaldosteronism 131 These conditions are the most frequently encountered abnormalities of adrenocortical function. In clinical practice they may constitute life-threatening medical emergencies requiring rapid diagnosis and institution of appropriate therapy. The following tests serve in the evaluation of the above conditions: Serum cortisol Urinary free cortisol ACTH in plasma Dexamethasone suppression test ACTH stimulation test CRH stimulation test Serum 17--hydroxyprogesterone BASIC DIAGNOSTIC CONSIDERATIONS The major adrenal corticosteroids are normally produced at the following rates: mg/day Cortisol 25 Corticosterone 2 Aldosterone 0.2 It is apparent that cortisol is the major steroid. The first diagnostic effort should establish the presence or absence of normal cortisol production by measuring the serum cortisol levels and urinary free cortisol. Serum Cortisol Cortisol is produced in the zona fasciculata of the adrenal cortex from cholesterol through a series of enzymatic reactions, the rates of which are controlled by ACTH. Only cortisol (and none of its precursors) when secreted into the bloodstream effects negative feedback on the hypothalamus and on ACTH release by the pituitary. ACTH release is further controlled by CRH (corticotrophin releasing hormone) from the hypothalamus and is subject to a diurnal rhythm related to the sleep-wake cycle and the influence of stress. Figure 1 ACTH release during a 24 hour cycle occurs in several bursts. The largest one is in the morning hours between 5 and 8 a.m. but others during the day appear related to food ingestion. Since the half-life of ACTH in circulation is only about 5 minutes while that of cortisol is about 65 minutes, the cortisol burst responses in the blood are less pronounced than those of ACTH and also lag behind. Cortisol serum levels are lowest from midnight to 4 a.m. and highest from 6 to 9 a.m. 132 (Figure 2). However, for practical reasons, sampling of blood for studying the diurnal rhythm is done at 8 a.m. and 4 p.m., the evening values normally being about half of the morning values. The total cortisol circulating in plasma is in mass equilibrium between proteinbound and free fractions such that 80% is bound firmly to transcortin (CBG, cortisol binding-globulin, an 1-globulin produced by the liver) and 10% is weakly bound to albumin. The remaining 10% is free. Only the free cortisol is metabolically active, and its level determines urinary excretion. The level of circulating transcortin rises in pregnancy and with estrogen therapy, resulting in an elevation of total blood cortisol; the free remains normal, since it is regulated by feedback mechanism. Transcortin normally is nearly saturated with bound cortisol. Thus, as in hypercortisolism, the binding capacity of CBG is exceeded when cortisol levels rise beyond 20 to 25 g/dL, resulting in a steep rise in free cortisol. The result is a sharp increase in urinary excretion of free cortisol while the rise in plasma cortisol is less dramatic. Methodology Blood cortisol is best determined by immunoassays. Although a major improvement over previous techniques, these methods are not entirely specific since corticosterone, cortisone, 11desoxycortisol, progesterone, and some synthetic steroids also bind in varying degrees to antibodies used in the assays. In conditions where these compounds may be elevated, this cross reactivity should be kept in mind. Figure 2: ACTH Release During a 24-hour Cycle Sample handling: Cortisol is a fairly stable substance, and clotted blood samples suffice when sent to the laboratory without delay. Reference Ranges: Morning values: 6-24 g/dL Evening values: 3-12 g/dL Interpretation of abnormal values: Increased values and particularly abolishment of diurnal variation are seen in Cushing’s syndrome. Increased values are also seen in pregnancy and 133 estrogen treatment (contraception) due to increase of CBG without functional hypercortisolism (see above), in alcoholism, in stress, and in starvation. Low values are seen in Addison’s disease, chronic illnesses, and in adrenogenital syndrome. D. URINARY FREE CORTISOL It would be desirable to measure the free cortisol in blood as this fraction constitutes the metabolically active form. However, this is technically not easily achieved. As the urinary excretion of cortisol parallels the level of free plasma cortisol, the former can be used as an indirect measure of the latter. As stated previously, a rise in plasma cortisol beyond about 20 g/dL, the approximate saturation level of transcortin, leads to a sharp increase of free cortisol and urinary cortisol excretion. For instance, a rise of plasma cortisol from 20 to 25 g/dL may be accompanied by a doubling of urinary free cortisol, or a rise from 20 to 40 g/dL in plasma may be accompanied by a five-fold rise in the urine. Thus, with urinary free cortisol determinations it is possible to differentiate more easily between normal and abnormally increased cortisol production as there is little overlap between the normal range and the range of values found in Cushing’s syndrome. In the diagnosis of Cushing’s syndrome, the sensitivity of this test is 96.9% and the specificity is 94.3%. Values seen in Cushing’s syndrome are almost always higher than 120 g/day. In fact, this test is most sensitive in the detection of hypercortisolism. Furthermore, in conditions of increased turnover of corticosteroids, as in obesity, or increases in transcortin, as in pregnancy, where the level of circulating free cortisol is not increased, the urinary free cortisol values remain normal. This test is not useful in the assessment of Addison’s disease, since a great deal of overlap of values occurs in the lower range of normal. Mild increases have occasionally been reported in starvation or in the use of topical steroids. 134 Reference Range: 20-90 g/day Precautions: a careful 24 hour urine collection is necessary; values may be low in renal disease with reduced glomerular filtration; determination of 24 hour urine creatinine excretion is of help to evaluate complete urine collection (1-2 g/24 hour) Figure 3: Hypothalamic-Pituitary-Adrenal Axis under Normal Conditions and in Various Adrenal Disorders (Tietz Textbook of Clinical Chemistry, 3rd edition, 1999, p. 1542) E. ADRENOCORTICOTROPHIC HORMONE (ACTH) The measurement of plasma ACTH would be a most useful diagnostic test in the evaluation of hyper- and hypocortisolism. Unfortunately, there are still technical and practical limitations which have prevented this test from becoming widely applied. ACTH is very unstable in blood samples because of its destruction by proteolytic enzymes within a few minutes; specimens should be centrifuged at 4C and immediately frozen. It is adsorbed onto glass and thus glass collection tubes should be avoided. The test, however, can have considerable diagnostic value if test conditions are rigidly controlled (addition of proteolysis inhibitors, immediate icing of blood samples, immediate handling by the laboratory during standard sampling times between 8 and 10 a.m.). Under these conditions normal subjects show serum concentrations ranging 50 to 100 pg/mL if a method is used that detects intact molecules and some fragments. The main application of this method is for detection of ectopic tumors. Values are 10-50 pg/mL if the method detects only intact ACTH. This method is preferred to evaluate pituitary ACTH secretion. The test is used: To differentiate primary from secondary adrenal insufficiency: in the primary form, because of the absence of the negative feedback on the pituitary by cortisol, ACTH secretion is not inhibited and levels are usually greater than 200 pg/mL. In the secondary form, due to pituitary insufficiency, levels are below 75 pg/mL. 135 To determine the cause of Cushing’s syndrome: in basophilic adenoma and ectopic ACTH production (as by oat cell carcinoma of the lung) levels may be higher than 200 pg/mL. In patients with adrenal adenomas or carcinomas that produce cortisol in excess, ACTH levels are low. In the demonstration of ectopic ACTH production by rapidly growing malignancies before the full-blown Cushing’s syndrome develops: values may be > 300 pg/mL. In the diagnosis of congenital adrenal hyperplasia: these patients lack cortisol and, therefore, have high ACTH levels. ACTH levels are expected to be in the normal range with adequate cortisol treatment. F. DEXAMETHASONE SUPPRESSION TEST This test is used in the differential diagnosis of Cushing’s syndrome. Its purpose is to determine whether the hypercortisolism that causes this syndrome is pituitary-dependent or not. Dexamethasone is a synthetic fluorine-containing cortisol analogue which has 10 to 20 times the metabolic potency of cortisol, including its negative feedback action on the pituitary. When given to patients in small test doses, dexamethasone inhibits ACTH release from the pituitary without contributing measurably to the total pool of glucocorticoids. Thus, under these conditions, dexamethasone does not interfere with plasma cortisol and urinary steroid measurements. Figure 4 Three versions of this test are in use: 1. The short (overnight) dexamethasone suppression test: this is a simple screening test only used to rule out Cushing’s. If depressed, the test is meaningful. Stress and obesity may invalidate the test, i.e. no suppression. A) Procedure: one (or two) mg of dexamethasone is given orally to the patient at 11:00 p.m., and at 8:00 a.m. a blood specimen is obtained for serum cortisol measurement B) Interpretation of results Serum cortisol less than 5 g/dL = normal Serum cortisol greater than 10 g/dL = possible Cushing’s syndrome. C) Advantages: simplicity; may be done on an outpatient basis 136 D) Disadvantages: false positives (nonsuppression) in psychiatric disease, stress, and induction of increased metabolism of dexamethasone in the liver by phenytoin (Dilantin); high cortisol values because of increased transcortin (pregnancy, contraception), and obesity; and of critically ill hospitalized patients, 25% will have a false positive result; false negatives are rare (< 1%). 2. The low-dose dexamethasone suppression test: the purpose of this test is the same as the short (overnight) test, but there are fewer false positives with this test. However, there are more false negatives (suppression in true Cushing’s) with this test than with the short test above. A) Procedure: a 0.5 mg dose of dexamethasone is given orally every 6 hours for 48 hours B) Interpretation of results Serum cortisol less than 5 g/dL = normal Serum cortisol greater than 10 g/dL = Cushing’s syndrome. 3. The high-dose dexamethasone suppression test: this test is performed as a followup after a low-dose test has shown no suppression. Its purpose is to distinguish adrenal hyperplasia caused by tumors of the pituitary (basophilic adenomas) from autonomous cortisol-producing adrenal adenomas and carcinomas, and ectopic carcinomas (i.e., oat cell carcinoma of the lung). The test is also helpful in distinguishing Cushing’s disease from ectopic ACTH secretion; the ectopic tumor generally resists negative feedback. A) Procedure: 24 hour urine collections are obtained daily for 4 days for free cortisol and 17-hydroxycorticosteroid determinations; Dexamethasone, 2.0 mg orally every six hours, is begun at 8 am on the second day and continued for eight doses; free cortisol and creatinine are measured in each twenty-four hour urine sample; other measurements include plasma cortisol at 8 a.m. and 8 p.m. on the first day to look for diurnal variation and at 8 a.m. on the fifth day B) Interpretation of results Patients with Cushing’s disease due to an ACTH-secreting pituitary adenoma usually show suppression of urinary free cortisol and 17-hydroxycorticosteroid excretion greater than or equal to 50% of baseline by the fourth day; lack of diurnal variation in plasma cortisol; and plasma cortisol 10 g/dL at 8 a.m. on the fifth day. Urinary free cortisol not suppressed = either autonomous production of cortisol due to adrenocortical adenoma or carcinoma or autonomous ACTH production ectopically by certain types of carcinoma. 137 C) Further differentiation Carcinomas of the adrenal cortex usually produce much larger amounts of steroids including androgens (testosterone) than do adenomas. Ectopic ACTH production sometimes can be demonstrated by selective venous blood sampling (via catheterization) from blood vessels draining tumor areas. G. ACTH STIMULATION TEST The purpose of this test is to assess the ability of the adrenal cortex to respond to ACTH stimulation by production of glucocorticoids. The test is used mostly in the differentiation of primary hypocortisolism (due to adrenal failure, i.e. Addison’s disease) from secondary hypocortisolism due to hypopituitarism. ACTH is a polypeptide chain composed of 39 amino acids. The biological activity of the hormone resides in that portion of the molecule comprising amino acid residues 1-24 (identical in all species so far examined). For medical use a synthetic ACTH consisting only of these 24 amino acids has been prepared. It has full biological activity and lacks antigenicity. The preparation mostly used in the U.S. is Cortrosyn. A short and a long version of the test are in use. 1. The short ACTH stimulation test A blood specimen is first drawn to obtain a baseline cortisol and then 250 g of Cortrosyn are given IV. Thirty and sixty minutes later, additional blood specimens are drawn for the stimulated cortisol levels. A normal response is a rise of at least 7 g/dL after 30 minutes and a rise of 11 g/dL after 60 minutes. If a normal response is obtained, nothing further needs to be done regarding assessment of adrenocortical function; the adrenal cortex has responded normally. If a subnormal response or no response is observed, the long stimulation test is indicated. In hypopituitarism of some duration the adrenal cortex is hypotrophic and needs stimulation over a longer period of time to respond. The test can be done on an outpatient basis. 2. The long ACTH stimulation test This test distinguishes primary from secondary hypocortisolism. Intravenous infusions of ACTH over 6 to 8 hours are given for 2 to 5 days. Urine free cortisol and serum cortisol are measured daily. Interpretation of results Serum cortisol values that are over 20 g/dL exclude primary adrenal insufficiency. Glucocorticoid withdrawal would be required before assessing secondary or tertiary adrenal insufficiency in such cases. Little or no increases in cortisol secretion are seen in primary adrenal failure even over successive days. A 138 progressive staircase rise is seen over 2 to 3 days in adrenal insufficiency caused by pituitary or hypothalamic disease or steroid level suppression. Little or no response is also seen in congenital adrenal hyperplasia due to 21- and 17hydroxylase deficiencies. Figure 5: Serum Cortisol Response to 250 g Synthetic ACTH (Tietz Textbook of Clinical Chemistry, 3rd edition, 1999, p. 1548) H. CRH STIMULATION TEST The CRH (corticotrophin releasing hormone) stimulation test may help to differentiate between Cushing’s disease and ectopic ACTH production. An intravenous bolus (100 g) of CRH results in an increase in plasma cortisol levels in normal individuals and patients with tertiary adrenal insufficiency (hypothalamic). Most patients with Cushing’s disease show normal or, frequently, exaggerated ACTH and cortisol responses to synthetic CRH, whereas patients with ectopic ACTH-secreting or adrenocortical tumors show no response. 139 Normal individuals: Cushing’s disease: peak serum cortisol occurs 60 minutes after injection and does not exceed 29.7 g/dL serum cortisol >29.7 g/dL or a rise of >25% above the basal level in patients with a basal serum cortisol >29.7 /dL I. SERUM 17--HYDROXYPROGESTERONE This test is used in the differential diagnosis of adrenogenital syndrome (congenital adrenal hyperplasia) to detect 21-hydroxylase deficiency. This enzyme converts 17-hydroxyprogesterone to 11-desoxycortisol in the pathway of cortisol biosynthesis in the adrenal. If the enzyme is absent, this biosynthetic step is blocked and 17-hydroxyprogesterone accumulates in high concentration in the blood (Figure 6). This condition may constitute a life-threatening medical emergency. The test can be done within a few hours (without having to resort to 24-hour urine collections) before steroid treatment is instituted. Reference values Less than 100 ng/dL In patients with 21-hydroxylase deficiency: greater than 1000 ng/dL J. ADDITIONAL TESTS FOR THE DIAGNOSTIC WORK-UP AND MANAGEMENT OF ADRENOCORTICAL DISEASE 1. HYPERCORTISOLISM (CUSHING’S SYNDROME) “Hypercortisolism” is now a generic term for elevated cortisol for any reason. Pituitary-dependent ACTH hypersecretion (Cushing’s disease) is the most common (70%) cause of endogenous Cushing’s syndrome in adults, followed by ectopic ACTH syndrome (12%). ACTH-independent causes of Cushing’s syndrome include adrenal adenoma (10%) and carcinoma (8%). If Cushing’s syndrome is suspected on clinical grounds (obesity limited to trunk, moon face, buffalo hump, abdominal purple striae, muscular weakness, osteoporosis, hypertension, dys- and amenorrhea, mental disturbances, hirsutism, virilization, diabetes mellitus), the suspicion can be further confirmed by obtaining electrolytes, serum glucose, SUN and urinalysis. 140 Electrolytes, Glucose, SUN, and Urinalysis Electrolytes: excess cortisol may have a mineralocorticoid effect leading to sodium and water retention, and potassium and hydrogen ion loss. The resulting picture is: normal sodium, low potassium, low chloride and high bicarbonate (hypokalemic alkalosis). In cases of ectopic ACTH production this may be seen before the full picture of Cushing’s syndrome develops. Glucose: the glucocorticoid effect of excess cortisol causes gluconeogenesis and impaired glucose tolerance. Glucose is high in 2/3 of cases. Urea nitrogen: SUN is moderately elevated because of increased protein breakdown leading to muscle wasting, osteoporosis and negative nitrogen balance. Urinalysis: glucosuria may be present 2. HYPOCORTISOLISM PRIMARY (ADDISON’S DISEASE) DEFICIENCY AND SECONDARY (PITUITARY) When hypocortisolism is suspected on clinical grounds (tiredness, weight loss, abdominal pain, nausea, hypotension, pigmentation of skin and buccal mucosa, mental disturbances) the suspicion can be further confirmed by obtaining serum electrolytes, glucose and SUN. Electrolytes, Glucose, SUN, and Urinalysis Electrolytes: in Addison’s disease, because of the lack of aldosterone, sodium is lost in the urine, and potassium and hydrogen ions are retained. The typical findings are that of hyperkalemic acidosis; sodium is low or normal (accompanying water loss), potassium is high, chloride is low, and bicarbonate is low. Glucose: glucose is low because of lack of glucocorticoid action, and there is increased insulin sensitivity. Urea nitrogen: urea nitrogen may be elevated because of hemoconcentration, volume depletion, and reduced glomerular filtration. For further confirmation, one should obtain plasma cortisol, morning value. If this value is high, hypocortisolism is excluded. Diagnosis of 21-Hydroxylase Deficiency A clinically severe and a milder form of this disease are observed. In the severe form there is marked sodium depletion since the 21-hydroxylase not only is involved in the biosynthesis of cortisol but also in that of aldosterone. Thus, these infants suffer from hypocortisolism and hypoaldosteronism with salt loss. Because the negative feedback control by cortisol on the pituitary is absent, ACTH production is unchecked and the 141 precursors prior to the enzymatic block accumulate. These precursors lead to increased production of androgens that are the causes of the anomalous genital development, virilization (pseudohermaphroditism) in girls, and precocious puberty in boys. In the clinically severe form, correction of the electrolyte abnormality and steroid replacement should be initiated without delay. Serum electrolyte and cortisol measurements should be obtained immediately in order to manage the acute condition. Blood and urine samples should also be collected prior to or at the beginning of treatment. Serum 17--hydroxyprogesterone may be elevated more than 10-fold, which is virtually diagnostic. If successful replacement steroid therapy is instituted, ACTH production by the pituitary is inhibited, and the abnormally high levels of serum 17-hydroxyprogesterone and urinary steroids decrease. This can be used as a guide to successful treatment. In the milder form the major abnormality is hypocortisolism while sufficient aldosterone is produced to prevent salt loss. However, virilization is still seen because of the overproduction of androgens. In some patients the only biochemical abnormality is an elevated serum level of 17--hydroxyprogesterone which is the diagnostic feature of this condition. These patients may be capable of producing sufficient cortisol for normal maintenance but are unable to increase cortisol when under stress. 3. HYPERALDOSTERONISM: The principal causes of primary aldosteronism (Conn’s syndrome) are adrenal adenoma, bilateral hypertrophy of zona glomerulosa cells and, rarely, adrenal carcinoma. If hyperaldosteronism is suspected on clinical grounds (hypertension, muscle weakness, latent tetany and paraesthesiae, polydipsia, polyuria), further investigation begins with obtaining serum electrolytes and urine potassium. Excess mineralocorticoids lead to sodium and water retention, and potassium and hydrogen ion loss. The resulting picture includes a high-normal sodium, low potassium, low chloride and high bicarbonate (hypokalemic alkalosis). In addition, inappropriately high urinary potassium excretion (normal <30 mmol/24h when serum K+ <3.6 mmol/L) is seen. Precautions: the serum and urinary potassium should be measured either before the patient starts treatment with hypotensive drugs or diuretics, or after the patient has been taken off such medications. In addition, one must be aware that primary aldosteronism may be mimicked by treatment with carbenoxolone and by ingestion of licorice (both have mineralocorticoid activity). For further confirmation, one should obtain: simultaneous measurement of plasma aldosterone and renin. This is an important test to distinguish between primary and secondary aldosteronism. In primary aldosteronism (Conn’s syndrome), plasma renin is reduced. In secondary aldosteronism (which is far more common than the primary form 142 and is associated with conditions such as cirrhosis, nephrotic syndrome and congestive heart failure), renin is high and is the cause of the excessive aldosterone secretion. Precautions: plasma aldosterone and renin are affected by posture and, therefore, basal blood measurements should be made with the patient recumbent. Bilateral adrenal venous sampling for measurement of aldosterone concentration remains the most accurate test in differentiating between an aldosteroneproducing adenoma and bilateral adrenal hyperplasia, although it can be technically difficult to perform. If the patient has an adenoma, the aldosterone is higher in the adrenal vein draining this gland than in the inferior vena cava, and lower (suppressed) on the contralateral side. In contrast, the aldosterone is higher in both adrenal veins than in the inferior vena cava in patients with idiopathic adrenal hyperplasia. 143 Cholesterol 20, 22-Desmolase Pregnenolone 17-Hydroxylase 3-Hydroxysteroid Dehydrogenase and 5-4-Isomerase* Progesterone 17-Hydroxypregnenolone 17-Hydroxylase 3-Hydroxysteroid Dehydrogenase and 5-4-Isomerase* 17-Hydroxyprogesterone Greatly increased urinary excretion of androgens (mainly androstenedione) and pregnanetriol 21-Hydroxylase 11-Deoxycortisol 21-Hydroxylase 11-Deoxycorticosterone 11-Hydroxylase Corticosterone 11-Hydroxylase 18-Hydroxylase and 18-Oxidoreductase* Cortisol Aldosterone Figure 6: Pathway of Cortisol Synthesis (Whitby LG, Smith AF, Beckett GJ and Walker SW, Lecture Notes on Clinical Biochemistry, 5th edition, Oxford: Blackwell Scientific Publications, 1993) The pathways in the synthesis of cortisol and aldosterone from cholesterol, showing the sites of the enzymic block in congenital adrenal hyperplasia that are due to the 21-hydroxylase defect, and the principal steroids that are excreted in abnormal amounts in this disease. * Single enzymes that possess both the catalytic activities that the names describe. 144 K. CASE STUDIES CASE 1 A 43-year-old woman has had fatigue, muscle weakness, facial puffiness, and easy bruising for 18 months. Physical examination reveals hypertension, round facies, muscle wasting, and numerous purpuric lesions. Plasma cortisol level (at 8:00 a.m.) is 32 g/dL. Urinary cortisol excretion is 475 g per day; it falls to 150 g per day after administration of dexamethasone, 8 mg daily. Chest and skull x-rays show osteopenia, without other abnormality. 1. What is the most likely cause (diagnosis) for these findings? 2. What additional studies should be performed? CASE 2 A 26-year-old woman had a normal pregnancy, but her delivery is complicated by uterine atony with excessive bleeding and shock. Five days after delivery she develops anorexia, nausea, vomiting, and confusion. At this time her blood pressure is 80/40 mmHg. The physical examination is otherwise unremarkable. The following laboratory studies are obtained: Hemoglobin 11.8 g/dL Leukocyte count 5200/L Serum Sodium 121 meq/L Potassium 5.2 meq/L Creatinine 0.4 mg/dL Glucose 56 mg/dL 1. What is the most likely diagnosis? 2. What additional studies should be performed? 145 CASE 3 A 52-year-old man has had general malaise and muscle weakness for 6 months. During the last 3 months he developed bilateral gynecomastia. For 2 months he has complained of feeling “fullness” and dull pain in his right abdomen and has noticed some weight loss. Laboratory data are: urinary free cortisol 350 g/day. Administration of 8 mg dexamethasone daily did not lead to a decrease in urinary free cortisol. 1. What is the most likely diagnosis? 2. What additional studies should be performed? 146 XI LABORATORY DIAGNOSIS OF THYROID DISEASE 147 148 A. OBJECTIVES To describe the most important hormones related to thyroid disease To describe the specific tests for thyroid diseases and how to interpret the laboratory data To list the factors that affect binding proteins To describe common diseases of the thyroid including Grave’s disease, Hashimoto’s thyroiditis, Plummer’s disease To describe neonatal thyroid screening B. KEY TERMS Exophthalmos - abnormal protrusion of the eyeball, also called proptosis Hashimoto’s disease - progressive autoimmune disease of the thyroid, patients gradually progress to hypothyroidism Hyperthyroxinemia - elevated concentration of thyroxine in blood Hypothalamus Pituitary Hypothyroxinemia - blood concentrations of thyroxine below the reference range Goiter - enlargement of the thyroid gland Grave’s disease - thyroid disease usually of autoimmune etiology with at least two of the following: hyperthyroidism, goiter, exophthalmos, generally caused by thyroid stimulating immunoglobin (TSI) Thyroid Plummer’s disease - functional adenoma of the thyroid, mutation that leaves TSH receptor activated, toxic multinodular goiter Thyroid stimulating hormone (TSH) - Secreted by the anterior pituitary in response to TRH, promotes growth, sustains and stimulates secretion of the thyroid gland Thyroid-stimulating immunoglobin (TSI) - an immunoglobin with agonist properties at TSH receptor Thyroiditis - inflammation of the thyroid gland Thyroxine - 3,5,3',5'-tetraiodothyronine (T4) Triiodothyronine - 3,5,3'-triiodothyronine (T3) 149 C. BACKGROUND/SIGNIFICANCE The thyroid gland secretes two iodine-containing hormones, thyroxine (T4) and triiodothyronine (T3). T4 is produced in a greater amount and is converted in peripheral tissues to T3, the more active hormone. The synthesis and secretion of the thyroid hormones is stimulated by thyroid-stimulating hormone (TSH), which is secreted by the anterior pituitary gland. The release of TSH is stimulated by the hypothalamic hormone, thyrotropin releasing hormone (TRH). T4 and T3 exert a negative feedback effect on the anterior pituitary and hypothalamus, and thus regulate TSH and TRH release, respectively. Since TSH is a good index of thyroid function it is recommended as the primary screening test. In the peripheral blood T4 and T3 are reversibly, and almost completely, bound to carrier proteins. These carrier proteins [thyroxine-binding globulin (TBG), T4 - binding prealbumin (TBPA), and albumin] bind 99.97% of T4 and 99.7% of T3. TBG is the major carrier protein. Due to the high binding affinities, only a very small fraction of the free, physiologically active hormones is in circulation. Wide variations in the concentration of binding proteins can occur, even under normal circumstances, and therefore there is variation in total T4 and T3 levels among euthyroid individuals, i.e. those having normal thyroid function. Thus, free T4 and free T3 provide a better indication of the patient’s thyroid status than total T4 and T3. Free T4 and free T3 provide verification of abnormal thyroid status indicated by the TSH screening test. D. TEST Table 1 is a list of serum tests used in the diagnosis of thyroid disease. Table 1 Adult Reference Range A. Total thyroid hormones thyroxine - T4 (rarely used) triiodothyronine - T3 4.5-10.5 g/dL 60-181 ng/dL B. Free thyroid hormones free T4 free T3 0.9-2.1 ng/dL 0.1-0.3 ng/dL C. Thyroid stimulating hormone (TSH) euthyroid hyperthyroid hypothyroid 0.35-5.5 mU/L* 0-0.35 mU/L > 5.5 mU/L * may be elevated in euthyroid elderly patients 150 Table 1 Adult Reference Range D. Anti-thyroid antibodies anti-thyroglobulin anti-microsomal antibody TSH-receptor antibody thyroid stimulating immunoglobulins (TSI) anti-thyroperoxidase antibodies negative negative none detected negative negative E. Reverse T3 20-80 ng/dL F. Thyroglobulin < 60 ng/mL E. CLINICAL APPLICATIONS 1. LABORATORY EVALUATION OF SUSPECTED THYROID DISEASE These most commonly encountered disease conditions and the tests used in their evaluation are: A) Hyperthyroidism: TSH, free T4, total T3, free T3, antithyroid antibodies, thyroid peroxidase antibodies, TSH-receptor antibody, thyroid-stimulating immunoglobulin (TSI), cholesterol. B) Hypothyroidism: TSH, free T4, antithyroid antibodies, cholesterol C) Goiter: TSH, free T4, antithyroid antibodies, total T3 D) Thyroiditis: TSH, free T4, antithyroid antibodies, total T3 E) Tumors: TSH, free T4, total T3, thyroglobulin F) TSH-secreting pituitary tumor (rare): TSH, free T4, total T3 2. CAUSES OF EUTHYROID HYPO- AND HYPERTHYROXINEMIA A) Causes of euthyroid hyperthyroxinemia Increased thyroid hormone binding o Acquired: pregnancy, newborn, liver disease (infectious hepatitis, chronic active hepatitis, primary biliary cirrhosis), acute intermittent porphyria, estrogen-producing tumors, hydatidiform mole, lymphosarcoma o Inherited abnormalities: increased TBG, albumin, prealbumin Peripheral resistance to thyroid hormones Transient hyperthyroxinemia of acute medical illness and acute psychiatric illness Drug related hyperthyroxinemia (exogenous estrogens, oral contraceptives, heroin, methadone,-fluorouracil, clofibrate) 151 B) Causes of euthyroid hypothyroxinemia Systemic illness - “sick euthyroid syndrome” Stress - physical and emotional Surgery (T3 decrease) Drugs (dopamine-TSH effect, salicylates, phenytoin, iodine, glucocorticoids, iodinated contrast agents, propranolol, amiodarone) Starvation and fasting (T3 decrease) Decrease in TBG 3. SIGNS AND SYMPTOMS OF THYROID DISEASE Signs and symptoms of hyperthyroidism include weight loss, tremors, tachycardia, cardiac arrhythmias, fatigue, heat intolerance, sweating, diarrhea, nervousness, irritability, scant menses in women, moist skin, hyperactive deep tendon reflexes, and enlarged neck Signs and symptoms of hypothyroidism (myxedema) include lethargy, weight gain, dry skin, puffiness (face and eyes), sparse hair, low body temperature, cold intolerance, fatigue, dizziness, voice hoarsening, depression, loss of libido, and occasional anemia A) Screening Case finding of hyper- or hypothyroidism in the general population: TSH with follow-up with free T4 or free T3, respectively. Abnormalities in thyroid function tests in euthyroid patients are most likely found in non-thyroid illness and the elderly. Thus, thyroid function tests should not be requested in elderly or hospitalized patients unless the present complaint suggests a thyroid problem. See Figure 4. B) Sick euthyroid syndrome (low T3 syndrome) The syndrome is seen in a variety of patients with non-thyroid illnesses, after acute injury, during caloric deprivation (especially carbohydrates), and in some psychiatric illnesses, e.g., schizophrenia. In these conditions, thyroid function test results may be different from normal, with the patient still being euthyroid. In most cases, the conversion of T4 in the peripheral tissues is not yielding T3, but reverse T3 (rT3). Therefore, a combination of low T3 and high rT3 is generally seen in this syndrome. Total T4 drops later in the disease to a variable degree. FT4 and FT3 may remain normal, but often they increase in the early part of the disease, and decrease later in the disease. No laboratory test is absolutely reliable in this condition, but the best test appears to be TSH. The incidence of the disease is higher than initially thought and may be as high as 50% in patients in the intensive care unit. 152 F. SPECIFIC TESTS AND INTERPRETATIONS 1. TOTAL THYROID HORMONES A) Thyroxine Total T4 is usually quantitated in serum by immunoassay. These assays offer the advantages of simplicity, speed, accuracy and precision. Since free T4 is not always available, one should understand the use of total T4. Figure 1: Thyroxine 3,5,3',5' Tetraiodothyronine (T4) Interpretation The reference range of total T4 has been established as 4.5 - 10.5 g/dL in serum. Values lower than 4.5 g/dL are indicative but not diagnostic of hypothyroidism. Workup should include a TSH, and free T4. Values higher than 10.5 g/dL are indicative but not diagnostic of hyperthyroidism. Workup should include TSH, and free T4. A finding of normal results does not absolutely exclude hyperthyroidism because elevations in T4 may be intermittent and/or hyperthyroidism may be due to an increase of total T3, which increases at times sooner than total T4. Elevations of the total T4 without hyperthyroidism are seen in: Increases of the TBG (see below) Full-term newborns up to about 6 weeks Familial dysalbuminemic hyperthyroxinemia in which an abnormal albumin preferentially binds T4 but not T3 and is characterized by elevations of total serum thyroxine although the patients are clinically euthyroid Decreases of the T4 without hypothyroidism are seen in: Decreases of the TBG (see below) Drugs that displace T4 from TBG (see below) B) 3,5,3'-Triiodothyronine (T3) The quantitative measurement of T3 in serum is primarily used to confirm hyperthyroidism and T3 thyrotoxicosis. Since free T3 is not yet widely available in the U.S., one should understand the use of total T3. Figure 2: 3,5,3' Triiodothyronine (T3) 153 Interpretation The reference range is from 65-180 ng/dL. Elevations or decreases of total T3 in serum generally parallel those of T4 in all respects regarding hormone production, TBG binding and drug interferences, and the interpretations of abnormal T3 values are the same as given above for total T4 with the following exceptions: The rare, so-called T3-thyrotoxicosis in which total T4 levels may be normal while T3 is elevated Starvation, including anorexia nervosa, where T3 may be low (high rT3) First days after birth, where T3 levels are low while T4 is high Sick euthyroid syndrome 2. ASSESSMENT OF FREE THYROID HORMONE LEVELS T4 and T3 are present in plasma in two forms: bound to plasma proteins and free. T3 is essentially bound only to TBG; however, T4 is also bound to prealbumin and albumin. The high affinities that govern these reactions result in the formation of 99.97% T4 bound and only 0.03% free T4; and 99.7% T3 bound, and 0.3% free T3. As stated above, T3 binds significantly only to TBG, while T4 is distributed between the 1-globulin TBG (75%), prealbumin (15%), and albumin (10%). Factors affecting binding proteins are shown in Table 2. Table 2: Factors that Affect Binding Proteins Binding Protein Increased Binding Protein Concentration or Binding Sites Decreased Binding Protein Concentration or Binding Sites Thyroxine-binding globulin Pregnancy Estrogens Oral contraceptives Infectious hepatitis Perphenazine (Trilafon) Acute intermittent porphyria Genetic (X-linked dominant) Androgens and anabolic steroids Nephrotic syndrome Large doses of glucocorticoids Active acromegaly Marked hypoproteinemia Genetic Phenytoin (Dilantin)* Salicylates* Prealbumin Prednisone therapy Severe illness or trauma Parturition Large doses of alicylates* * These agents affect binding capacity by displacing hormone from the proteins. In clinical practice, the most frequently encountered findings are due to pregnancy or oral contraception. 154 It is well established that the thyroid state of the patient correlates better with the plasma concentrations of the free forms of T4 and T3 than with the levels of total hormone concentrations. Thus, a most important measurement in the assessment of thyroid status is an estimation of free T4 and free T3. The direct accurate measurement by dialysis or ultrafiltration of the free T4 and free T3 is the “gold standard” measurement but the procedure is difficult and time consuming, and thus impractical for routine use. For these reasons, simpler methods have been developed that estimate the free hormone levels by indirect means. The most commonly used procedure is the two-step fluorometric enzyme immunoassay for free T4. The most reliable procedure is the dialysis technique. 3. THYROID-STIMULATING HORMONE [TSH (THYROTROPIN)] Thyrotropin is a glycoprotein of a MW 28,000. It is composed of two noncovalentlylinked subunits termed and . The -subunit of TSH is identical to the -subunits found in FSH, LH and hCG, whereas the -subunits in these peptide hormones are distinctly different and account for their biological and immunological differences. The quantitative ultrasensitive immunometric TSH determination in serum is the most useful test to detect hypo- and hyperthyroidism. Additionally, serum TSH is a very useful test in distinguishing primary (thyroidal) and secondary (pituitary) hypothyroidism. TSH is elevated in primary hypothyroidism when there is a defect in thyroid hormone production, whereas in secondary hypothyroidism serum TSH levels are low due to pituitary failure. TRH - TSH Stimulating Test A TRH- (thyrotropin-releasing hormone) TSH stimulating test is useful for determining pituitary function since administration of TRH will normally increase plasma levels of TSH within 30 minutes after administration, whereas no increase in plasma TSH is observed in pituitary hypothyroidism. A flat response to TRH is observed in hyperthyroidism and presumed subclinical hyperthyroidism (also seen in pituitary TSH deficiency). Tertiary (hypothalamic) hypothyroidism results from deficient hypothalamic synthesis of TRH. Since there is no available reliable assay for serum TRH, hypothalamic malfunction can be inferred from a TRH stimulation test. See Figure 3. 4. ANTITHYROID ANTIBODIES High titers of anti-thyroglobulin and anti-microsomal antibodies are found in autoimmune diseases such as chronic lymphocytic (Hashimoto’s) thyroiditis (hypothyroid cases) and lymphadenoid goiter (euthyroid cases). Recent evidence indicates that sera with antimicrosomal autoantibodies from patients with Grave’s disease or Hashimoto’s thyroiditis bind to thyroid peroxidase, suggesting that thyroid peroxidase is the microsomal antigen in these diseases. 155 Figure 3: Patterns of TSH Response to TRH Stimulation (Tietz Textbook of Clinical Chemistry, 3rd edition, 1999, p. 1548) 5. THYROID STIMULATING IMMUNOGLOBULINS (TSI) IgG human thyroid stimulators are present in nearly all cases of Grave’s disease when the assay is performed with human thyroid cells or cell membranes. Thus, it appears that a human thyroid stimulator immunoglobulin is the mediator for the expression of Grave’s disease and that this antibody mimics the action of TSH by combining with the TSHreceptor sites of the thyroid cells. However, TSI does not respond to the feedback mechanism and thus continuously stimulates secretion of thyroid hormones. 6. REVERSE T3 (rT3) Reverse T3, 3, 5, 5'-triiodothyronine (rT3), is an inactive metabolite of T4 that may be increased in acute febrile illnesses, chronic hepatic cirrhosis, and other miscellaneous chronic systemic illnesses, and particularly in newborns. In these patients, low T3 concentrations are found, (although they are euthyroid) because T4 is deiodinated to rT3 rather than T3 and it may be useful to confirm this by an rT3 assay. rT3 measurements in amniotic fluid have been proposed as a test for assessing fetal thyroid function because rT3 is the major metabolite of T4 in the fetus. The serum reference ranges for rT3 in adults and children are 30 - 80 and 20 - 70 ng/dL, respectively. 156 7. THYROGLOBULIN Thyroid tissue is the sole source of thyroglobulin (MW = 660,000) in peripheral blood. Patients with thyroid cancer who have been thyroidectomized should have no circulating thyroglobulin, and increases in this iodoprotein are generally indicative of tumor recurrence. G. SCREENING NEONATAL THYROID SCREENING Congenital hypothyroidism occurs in one out of every 4,000 newborn infants. Early detection of neonatal hypothyroidism is important since mental retardation that results from this disease can be prevented by prompt thyroid hormone treatment. Therefore, a sensitive RIA for T4 using a 3 mm spot (equal to 3 L) of whole dried blood on filter paper has been developed to detect hypothyroidism in 3-5 day old infants. If the plasma T4 concentration is below 7 g/dL, a follow-up TSH, that utilizes the whole dried blood sample on filter paper, is performed to establish a definitive diagnosis of primary hypothyroidism. Blood is collected from neonates by heel puncture. Blood spots on filter paper can be sent by mail, and portions of the same spots can be utilized for the phenylketonuria screening test. In neonates, thyroid hormone levels and TBG are higher than in adults. Table 3: Thyroid Hormone Levels in Neonates T4 T3 (ng/dL) (g/dL) Cord serum 10.9 (7.7 - 14.1) 48 (16 - 80) Age 3 days 13.3 (8 - 19) 125 (45 - 205) Age 6 weeks 10.3 (7.3 - 13.3) 163 (119 - 209) After 1 year adult levels ADULT POPULATION SCREENING: CASE FINDING GENERAL POPULATION OF UNSUSPECTED THYROID DISEASE IN The prevalence of thyroid disease in the general population is estimated to be 1-2%. Thus, thyroid disease constitutes one of the most frequent diseases that is amenable to treatment. For this reason, screening for thyroid disease appears advisable and is best done by including a TSH and FT4 determination in biochemical panels to examine well populations and patients that seek medical attention for reasons other than their thyroid status. Remember the complications in interpretation of thyroid tests caused by nonthyroidal illness and advanced age. 157 SUBCLINICAL HYPERTHYROIDISM15 The combination of an undetectable TSH (<0.1 mU/L) and normal serum T3 and fT4 (usually at the upper end of the reference range) is known as subclinical hyperthyroidism. An absence of symptoms was once part of the definition but we now understand that subtle symptoms or signs of thryotoxicosis may be present. The prevalence of subclinical hyperthyroidism is about 2%. The health consequences of overt hyperthyroidism are well known to include atrial fibrillation and osteoporosis. While the effects are much less evident in subclinical hyperthyroidism it does bear some discussion. Subclinical hyperthyroid patients have a relative risk of 3 for atrial fibrillation as compared to those patients with normal TSH; however, a low but detectable TSH of 0.1 to 0.4 mU/L did not have an increased risk of atrial fibrillation. The case is not as clear for osteoporosis. Subclinical hyperthyroid patients that have endogenous sources of thyroid hormones appear to have increased bone turnover. Most impressive is the reported loss of 2% bone mineral density per year in postmenopausal women with multinodular goiter. On the other hand, patients with exogenous sources of excess thyroid hormone the risk factor is inconclusive. The 2004 expert panel on thyroid disease recommends observing and monitoring patients with partial TSH suppression (0.1 to 0.4 mU/L) but to treat patients with complete TSH suppression (<0.1 mU/L).16 SUBCLINICAL HYPOTHYROIDISM16-17 Subclinical hypothyroidism is defined as elevated TSH associated with normal concentration of T3 and fT4. The overall prevalence is 5-10% in the general population and up to 20% in women over 60 years old. While the consensus panel recommends treatment of patients with TSH >10 mU/L, there is some controversy with the treatment of patients with TSH of 4.5 to 10 mU/L. The key determinate is the clinical judgment of the health care provider. Thyroid failure can be considered a continuum with patients having TSH of 4.5 to 10 mU/L being on one end of the spectrum and those with myxedema coma on the other end. Milder disease would be related to lesser adverse effects; however, the intervention must be also evaluated for potential harm. About 20% of patients receiving levothryoxine are overtreated. This should not argue against treatment - instead it is an important statement endorsing laboratory monitoring to insure that the resulting thyroid hormone treatment is reflected in an appropriate TSH. 15 Toft AD, Subclinical hyperthyroidism [Clinical Practice], N. Engl. J. Med. 345:512-516, 2001 Hossein G et al., Subclinical thyroid dysfunction: a joint statement on management from the American Association Of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society, Endocr. Pract. 10:496-501, 2004 17 Cooper DS, Subclinical hypothyroidism [Clinical Practice], N. Engl. J. Med. 345:260-265, 2001 16 158 Treatment should not be a reflexive action based on a solitary TSH measurement but should be considered in the overall clinical situation. It is important to ensure that abnormal TSH values are not a transient adjustment in progress due to other treatments or responses to illness. Figure 4: Strategies for Thyroid Diagnosis using a Sensitive TSH Assay as The Initial Step (Tietz Textbook of Clinical Chemistry, 3rd edition, 1999, p. 1505) 159 H. CASE STUDIES CASE 1 A 27-year-old, non-pregnant woman has a serum T4 of 13.5 g/dL and a serum T3 of 280 ng/dL. Her serum TSH, cholesterol, and CK were all normal, and her physical examination was within normal limits. 1. What is the most likely explanation for these findings? 2. What other thyroid function tests should be obtained to confirm your conclusion? CASE 2 A 59-year-old woman complaining of numbness and paresthesia of her right index and ring fingers had gained about 30 pounds the previous year. She exhibited a tired look with slight periorbital puffiness and a diffusely enlarged thyroid (1.5 times normal size). For the last six months she had noted dry skin, decreased energy and a change in her voice. Her heart sounds were decreased, but there was no cardiac enlargement. Her heart rate was 56 beats per minute, and blood pressure was 130/90 mmHg. The remainder of her physical exam was unremarkable except that the relaxation phase of her deep tendon reflexes was delayed. Laboratory Findings Serum T4 2.6 g/dL Free T4 0.5 ng/dL Serum TSH 110 mU/L Serum cholesterol 375 mg/dL Creatine kinase 425 U/L (no increase in MB isoenzyme) Antimicrosomal antibodies in serum positive at 1:410,000 dilution Antithyroglobulin antibodies in serum negative 1. What is the most likely diagnosis? 2. List other etiologies for hypothyroidism. 160 CASE 3 A slightly anxious 49-year-old woman with bright eyes, but no exophthalmos, presented with a slight tremor and a diffusely enlarged thyroid gland. Her serum T4 was 19.8 g/dL, free T4 4.2 ng/dL and T3 660 ng/dL. The serum was positive for antimicrosomal and antithyroglobulin antibodies. 1. What is the diagnosis for this patient? 2. Would it be useful to obtain serum TSH levels? 3. What additional test might be helpful? 161 CASE 4 A 55-year-old man complained of nervousness and fatigue which had been apparent for 3 months. He had lost 10 pounds despite a voracious appetite. Recently, he had been bothered by palpitations and shortness of breath. All of his symptoms seemed heightened by the summer heat which caused him to perspire excessively. About a month ago, he noted redness and tearing of both eyes. On examination, he is a thin, anxious appearing man, 68 inches in height, whose weight is 155 lb. Pulse is 110 BPM and blood pressure is 140/70 mmHg. There is bilateral proptosis, periorbital edema, and marked conjunctival congestion. He is unable to move his eyes completely into the superior-temporal position. Visual acuity and fundoscopic exam are otherwise normal. The thyroid gland is moderately firm and symmetrically enlarged to an estimated 50 g (normal, 15-20 g) without any palpable nodules. A systolic bruit and lateral venous hum are heard over the gland. The skin is warm and smooth, and several areas of vitiligo are prominent on his hands and feet. Hair is of fine texture. Reflexes are brisk, and there is a fine tremor of the outstretched hands. The following serum values were reported (reference values in parenthesis): Serum TSH T4, total T4, free Antithyroglobulin antibodies Antimicrosomal antibodies Thyroid-stimulating immunoglobulin (TSI) Cholesterol Calcium, total Phosphorus Alkaline phosphatase Glucose Reported Results < 0.1 U/mL 14.2 ug/dL 3.8 ng/dL negative positive, 1:1280 positive 120 mg/dL 5.4 mEq/L 4.9 mg/dL 160 U/L 125 mg/dL Reference Range (0.5-4.6) (4.6-10.5) (0.7-2.0) (negative) (negative) (negative) (140-220) (4.2-5.1) (2.7-4.5) (49-120) (75-105) The 24 hour radioactive iodine uptake is 68% (5-28%). Thyroid scan shows enlargement with a diffuse uptake. The patient is treated with propylthiouracil (PTU). After 3 weeks of therapy, the patient complains of a sore throat. CBC reveals a diminished white count. PTU is discontinued and alternate therapies are considered. 1. Based on the history and physical, what is your initial clinical impression? 2. What are some possible causes of this disorder? 162 3. What screening tests support your initial clinical impression? 4. What is the significance of the positive TSI and abnormal radioiodine uptake? 5. What is the pathogenesis (and morphology) of this patient’s disease? 6. What therapies are available to manage this patient? 7. What is “thyroid storm”? How is it treated? 8. Why are Ca, P, and alkaline phosphatase increased? 163 164 XII LABORATORY DIAGNOSIS OF LIVER DISEASE 165 166 A. OBJECTIVES To describe the basic functions of the liver To describe how to use the laboratory to determine if liver disease is present To describe how to use the laboratory to determine what type of liver damage is present (cellular vs. biliary tract) To list the various tests used to diagnose hepatitis B. KEY TERMS Alkaline phosphatase - enzyme found in a variety of tissues; commonly used to diagnose hepatic and bone diseases AST - aspartate aminotransferase; commonly used to detect cellular hepatic damage; sometimes referred to as SGOT ALT - alanine aminotransferase; commonly used to detect cellular hepatic damage; sometimes referred to as SGPT Bilirubin - end product of heme metabolism produced in liver, spleen and bone marrow Cholestasis - decreased flow of bile Cirrhosis - fibrosis or scarring of liver Direct bilirubin - bilirubin that has been conjugated with glucuronic acid GGT - gamma glutamyl transferase; useful to help determine if elevations of alkaline phosphatase are due to bone or liver Hepatitis - inflammation of the liver Indirect bilirubin - bilirubin that has not been conjugated C. BACKGROUND SIGNIFICANCE In the liver, important synthetic, catabolic, and detoxifying functions take place. Complete loss of the liver or its functions results in death within hours or days. The hepatic parenchymal cells are involved in carbohydrate metabolism (glycogen synthesis, glycogenolysis, gluconeogenesis), protein synthesis (most plasma proteins including the coagulation factors, except factor VIII and immunoglobulins), lipid metabolism, (lipoproteins, phospholipids, fats, cholesterol), storage functions (probably all vitamins except vitamin C, iron, copper, trace metals), excretion (bilirubin, bile salts, copper), and detoxification (ammonia, steroid hormones, drugs, poisons). Liver disease may be a process confined to the organ itself (e.g., infection, cirrhosis, cholestasis, neoplasms) or be a consequence of systemic disease (e.g., cardiac insufficiency, hemochromatosis, hemolytic disease, metastatic disease). 167 Many drugs are cleared by hepatic mechanisms, and many drug interactions occur at the hepatic level. In addition, several drugs cause hepatic damage, consequently it is essential for pharmacists to understand how to use the laboratory to: 1. Assess the state of biochemical functions of the liver 2. Differentiate pathologic processes from drug-induced effects 3. Diagnose etiology 1. TESTS OF HEPATIC PARENCHYMAL DAMAGE A) Tests of hepatocellular damage AST 10-30 U/L ALT 11-45 U/L Bilirubin (conjugated and total) <0.2 and <1.2 mg/dL, respectively B) Tests of biliary tract disease Alkaline phosphatase (ALP) 30-130 U/L Gamma glutamyl transferase (GGT) o female <24 U/L o male <38 U/L Bilirubin (conjugated and total) < 0.2 and < 1.2 mg/dL, respectively 2. TESTS OF ETIOLOGY Hepatitis virus antigens; HBsAg, HCV by PCR Hepatitis virus antibodies (e.g., HAV-IgG, HAV-IgM, HBcAb, HBeAb, HBsAb, HCAb) -fetoprotein Immunoglobulins Autoantibodies (antimitochondrial, smooth muscle, antinuclear) Iron, transferrin, ferritin (hemochromatosis) Copper, ceruloplasmin (Wilson’s disease) D. DESCRIPTION OF TESTS 1. BILIRUBIN Reference Range: Conjugated bilirubin <0.2 mg/dL Total <1.2 mg/dL Bilirubin, the end product of heme catabolism, is transported to the liver to be conjugated and excreted via the bile. Most of the bilirubin in blood is in transit from the tissues to the liver, in the unconjugated form, bound to albumin. Only small amounts of conjugated bilirubin are normally found in blood, and it is believed that the usual analytical methods tend to overestimate it in the low reference range. Bilirubin determinations are reported in two fractions, the “conjugated” and the “total.” Conjugated bilirubin refers to bilirubin glucuronide. Total bilirubin refers to the amount of conjugated plus unconjugated bilirubin. Unconjugated bilirubin conventionally is neither measured nor reported as 168 such, but in practice is inferred by the clinician from the difference (total bilirubin minus the conjugated fraction). Hemolysis interferes with the test, causing an erroneous lowering of results (depending on methodology). Bilirubin is very light sensitive and samples must be analyzed within one to two hours or kept in the dark. Bilirubin is stable for months when serum is frozen, and protected from light. Fasting increases bilirubin in blood slightly in the normal person and more markedly in Gilbert’s disease (see section below). It decreases with sunbathing and is slightly lower in women than in men. Low bilirubin values have no demonstrated clinical significance. Elevations of the unconjugated bilirubin without significant elevations of the conjugated are seen in: Hemolytic disorders Gilbert’s syndrome (transport and conjugation defect, bilirubin 2-3 mg/dL) Arias syndrome (partial conjugation defect, bilirubin more than 6 mg/dL) Crigler-Najjar syndrome (glucuronyl transferase deficiency; 2 types) Immaturity of the liver in the newborn Cirrhosis (often no elevations) Elevations of the conjugated bilirubin with lesser increase of the unconjugated form are seen mostly in the early phases of cholestasis and are transient, with day to day fluctuation of the two fractions. Textbook descriptions of conditions typically characterized by an elevation of the conjugated bilirubin often neglect to mention that these conditions are also accompanied by an elevation of the unconjugated bilirubin (e.g., Dubin-Johnson, Rotor). Elevations of the conjugated plus unconjugated bilirubin are seen in: Hepatitis Cirrhosis Posthepatic obstruction Dubin-Johnson syndrome - young women on contraceptives or pregnant, usually related to estrogen-induced inability to excrete bilirubin (liver pigmentation; gallbladder not visualized; excretory defect) Rotor’s syndrome (no liver pigmentation; gallbladder visualized; excretory defect) Some forms of drug-induced cholestasis A third form of bilirubin “tightly” bound to plasma albumin has been characterized by high performance liquid chromatography (HPLC). This form of bilirubin (“deltabilirubin”) is not detected to any significant extent in sera from normal adults, or in sera from patients with unconjugated hyperbilirubinemia. However, increased levels of deltabilirubin are observed in sera from patients with prolonged conjugated hyperbilirubinemia (e.g., patients with cholestasis, cirrhosis, hepatitis, Dubin-Johnson 169 syndrome, biliary atresia of the newborn). It has been suggested that a spontaneous reaction between bilirubin glucuronides and albumin results in a covalent linkage, presumably an amide bond, of pigment to the serum albumin. The concentration of deltabilirubin varies over a wide range, but it may account for 30-50%, and in some instances as much as 90%, of the total serum bilirubin. Delta-bilirubin is believed to be responsible for persistence of conjugated hyperbilirubinemia following clinical disappearance of bilirubinuria. Delta-bilirubin per se is not cleared into the urine since it is bound to albumin. Bilirubinuria Only the conjugated, water-soluble form of bilirubin (the direct) is excreted into the urine. Since the conjugated bilirubin concentration in serum normally is very low, the urine normally also contains less than 0.2 mg/dL of bilirubin, an amount not detectable by conventional tests. Thus, detectable bilirubinuria is abnormal and for its demonstration qualitative or semi-quantitative tests suffice. Bilirubinuria is seen in: a. Biliary obstruction b. Hepatocellular damage c. Dubin-Johnson syndrome d. Rotor’s syndrome In conditions with an elevation of only unconjugated bilirubin, bilirubinuria is not present. 2. PLASMA PROTEINS Plasma total protein concentrations in liver disease often are near normal because a decrease of albumin is offset by an increase of globulins and the concept of the ratio of albumin:globulin (A/G ratio), as used in the past, reflects this. Reference Range: 1.5:1 to 3.0:1. Most useful are albumin measurements in the assessment of the severity of impairment of the synthetic functions of the liver. Chronic proliferative disease of the liver (cirrhosis, chronic hepatitis) leads to polyclonal hypergammaglobulinemia as demonstrated by the “beta-gamma bridging” on serum protein electrophoresis. This is due to immune responses in these conditions. Albumin is also important in the evaluation of renal disease. 3. BLOOD COAGULATION A prolongation of the prothrombin time (PT) is an indicator of hepatic dysfunction as the synthesis of the coagulation factors is impaired in hepatocellular disease. Furthermore, impaired absorption in the gut, particularly of fat soluble substances, often accompanies liver disease which may result in decreased absorption of vitamin K, needed for the 170 production of factors II, VII, IX and X. In more severe conditions, the PTT (partial thromboplastin time) also becomes prolonged. Dysfibrinogenemias, an interferent with fibrin-polymerization, have also been reported in severe liver disease. Fibrinolysis is increased in some patients with hepatic disease, presumably because of reduced synthesis of plasmin inhibitors by the diseased liver. 4. AMINO ACIDS In severe cirrhosis or hepatitis, the handling of amino acids by the liver cell is impaired, resulting in higher blood levels and increased aminoaciduria. There is little clinical value in their determination. In severe liver necrosis, aminoaciduria reaches such proportions that the solubility of certain amino acids is exceeded and crystals are formed, such as the characteristic leucine “spheres” and tyrosine “rosettes” (see under Urinalysis) which are encountered in the urine in “acute yellow liver atrophy.” 5. BLOOD AMMONIA Elevated blood ammonia is seen in severe liver disease and in actual or impending hepatic coma. The elevations are due to: Reduced removal of ammonia from the portal blood, and “shunting” of portal blood, bypassing the liver Blood ammonia levels show some, but not close, correlation with the presence and deepness of the coma 6. AST (ASPARTATE AMINOTRANSFERASE) AND ALT (ALANINE AMINOTRANSFERASE) These transaminases are the most sensitive indicators of hepatic cell injury. They may be elevated without elevation of bilirubin or other detectable impairment of liver function. Their highest levels are seen in acute hepatic necrosis. Their elevations usually parallel each other. ALT elevations without those of AST are occasionally seen in mild hepatic disease and in the recovery phase of hepatitis, as the ALT elevations persist longer than those of AST. ALT is generally a more sensitive indicator of acute liver cell damage than AST. 7. ALKALINE PHOSPHATASE (ALP) This enzyme is present in high concentration in the lining of the biliary system (bile canaliculi) and escapes into the bloodstream when the lining cells are affected by inflammation, necrosis, or obstruction. In obstruction, marked increase in serum concentrations are probably due to release of the enzyme into the circulation as a result of cell fragmentation by the increase of bile acids. 171 Elevations of this enzyme in the blood are a sensitive indicator of a biliary process but they are also seen in liver cell damage. The general rule applies that the higher the alkaline phosphatase, the greater the chances for post-hepatic obstruction. If the elevation is less than three times normal, consider hepatocellular disease; if greater than three times the upper reference limit, consider post-hepatic obstruction. Focal lesions in the liver may lead to significant elevations of the alkaline phosphatase without a raised bilirubin. The liver has a large functional reserve and in this situation the unaffected liver tissue has sufficient capacity to excrete the bilirubin, while the alkaline phosphatase remains circulating in the blood. It must be kept in mind that there are other important sources of alkaline phosphatase such as the bones and the gastrointestinal mucosa, the placenta, and certain tumors (Regan and Nagao isoenzymes). The alkaline phosphatases show differential heat stability when exposed to 56 or 60C in the following order: bone < intestine < liver < placenta, Regan isoenzyme (most heat stable) and the heat stability test of alkaline phosphatase takes advantage of this. Remember this by “bone burns and liver lives” and “BILP.” In 1994, Hybritech introduced a specific test, Ostase®, for the bone isoenzyme. The only limiting factor is a ~12% cross-reactivity with the liver isoenzyme. 8. GGT (GAMMA GLUTAMYL TRANSFERASE) This enzyme is found in liver and pancreas, but in even larger amounts in the kidney. Elevations of the hepatic alkaline phosphatase can be due to a bone disease or due to cholestasis. Therefore, GGT determinations are helpful in differentiating bone and liver sources of alkaline phosphatase, since there are no significant amounts of GGT in bone. GGT is also a sensitive indicator of alcohol-induced liver disease and of recent alcohol ingestion. 172 E. CASE STUDIES CASE 1 (Ann. Pharmacother. 36(12):1887-1889, 2002) A 41-year-old white man had received a prescription for celecoxib 200 mg/d for 3 days for pain associated with right-knee trauma. Shortly after taking the second dose of the drug, he began to experience epigastric pain, nausea, anorexia, malaise, pruritus, cutaneous and scleral jaundice with dark urine, and pale stools. In spite of the symptoms, the man took the final dose (total dose: 600 mg). The symptoms persisted and, 48 hours after the third dose of celecoxib, he was seen by his family physician, who found: a total bilirubin 8.4 mg/dL, direct bilirubin 7.6 mg/dL, AST 97 IU/L, ALT 234 IU/L, and GGT 134 IU/L. 1. How do you interpret these lab values? 2. What is the significance of the pale stools? 3. What other tests should be ordered? 4. Is this a common adverse drug reaction for this class of drugs? 5. What percent of patients that present with cholestasis are drug-induced? 6. How should this patient be treated? 173 CASE 2 (Am. J. Med. Sci. 325(5):292-295, 2003) A previously healthy 65-year-old man developed scleral jaundice. Simultaneously, he experienced severe fatigue, and reported light-colored stools, dark urine, and generalized pruritus. The patient denied abdominal pain, fever, abnormal bleeding, or skin rashes. One month before the onset of symptoms, he was prescribed terbinafine (Lamisil; Novartis Pharmaceuticals Corp., New Jersey) for onychomycosis (fungal infection of the nails). Initial laboratory tests showed bilirubin 21 mg/dL, (conjugated bilirubin, 18.8 mg/dL), aspartate aminotransferase 313 IU/L (reference range: 8-58 IU/L), alanine aminotransferase 179 IU/L (reference range: 8-52 IU/L), and alkaline phosphatase 1040 IU/L (reference range: 34-124 IU/L). 1. How do you interpret these lab values? 2. What other lab tests should be ordered? 3. Is this a common ADR for this drug? 4. How should this patient be treated? 174 CASE 3 (Am. J. Med. Sci. 325(1):31-33, 2003) An 80-year-old man had been diagnosed with hypopharyngeal cancer and received radiation therapy. He had an episode of acute bronchitis, for which he was given 500 mg of oral amoxicillin/clavulanic acid (Augmentin). Because of persistent upper respiratory symptoms and fever over the subsequent 2 days, 500 mg of oral ciprofloxacin twice a day was added. Within 48 hours, the patient’s fevers began to subside, with an improvement in respiratory symptoms. Six days later, the patient developed a generalized maculopapular, intensely pruritic rash. Pertinent laboratory studies showed total bilirubin of 0.8 mg/dL, aspartate aminotransferase (AST) of 150 U/L, alanine aminotransferase (ALT) of 154 U/L, alkaline phosphatase of 120 U/L, serum albumin of 2.1 g/dL, and prothrombin time of 13.9 seconds. Four days later the liver profile showed an AST of 577 U/L, ALT of 972 U/L, ALP of 358 U/L, total bilirubin of 1.9 mg/dL, direct bilirubin of 1.1 mg/dL, and GGT of 638 U/L. 1. What is the interpretation of these lab values? 2. Propose a mechanism for these observations. 3. What is the mechanism of action of Augmentin? 4. Is this a common ADR for these compounds? 5. How should this patient be treated? 175 176 XIII TUMOR MARKERS 177 178 A. OBJECTIVES To understand the primary uses and limitations of tumor marker assays To describe which tumor markers are indicated in monitoring various neoplasms B. KEY TERMS Adenoma - benign epithelial tumor Benign - not malignant, not recurrent, favorable for recovery Cancer - a relative autonomous growth of tissue Malignant - tending to become progressively worse, having properties of invasion and metastasis Metastasis - transfer of disease from one organ to another not directly connected to it Sarcoma- connective tissue tumor, most are malignant Tumor - new growth of tissue which is uncontrolled C. BACKGROUND SIGNIFICANCE Cancer is the second most common cause of death in the United States. Many strategies are employed for early diagnosis and monitoring of therapy. Tumor markers’ primary use is limited to detecting recurrence and monitoring therapy, but are sometimes used as screening tests. It is important that clinicians understand how sensitivity, specificity and prevalence impact the use of tumor markers. D. USE AND LIMITATIONS OF TUMOR MARKERS 1. TUMOR MARKER Definition: a substance produced by a tumor or by a host in response to the tumor’s presence; such substances can be measured in blood, fluid secretions, or tissues by chemical, immunochemical, and molecular biology methods or by cytochemical staining Classifications: markers include enzymes and isoenzymes, specific proteins, hormones, oncofetal antigens, carbohydrate epitopes, receptors, metabolites, and genetic changes 2. USE OF TUMOR MARKERS 1. For screening populations and high-risk groups 2. As an aid in diagnosis (limited value; lack of specificity and sensitivity) 3. In staging or confirmation of histopathology 4. In therapy to monitor drug response 5. To check for recurrence (main use) Their use is mainly in carcinoma, less so in sarcoma. 179 3. EXAMPLES OF TUMOR MARKERS TEST & SOURCE CLINICAL USE AFP (-fetoprotein) A tumor-associated protein. Increased in non-seminomatous testicular cancer; useful for monitoring its clinical management. Serum Also increased in primary hepatocellular cancer and germ-cell tumors of yolk-sac origin. CA 19-9 (Cancer Antigen 19-9) Serum CA 125 (Carbohydrate Antigen 125) Serum A tumor-associated glycoprotein. Increased in 80% of patients with pancreatic adenocarcinoma but rarely in patients with benign pancreatic disease. Useful in monitoring post-operative patients for disease recurrence. Tumor-associated glycoprotein. Increased in most patients with epithelial ovarian cancer. Should be used as an aid in the detection of residual ovarian cancer in patients who have undergone firstline therapy and should be considered for diagnostic second-look procedures. FDA approved for this purpose only. Carcinoembryonic A glycoprotein. Increased in a variety of malignancies, Antigen (CEA) predominantly colorectal cancer, but also CA of pancreas, breast, cervix, uterus, ovary, bladder, and lung. Useful for monitoring Serum patients for disease recurrence. Persistently elevated levels or increasing levels may reflect the presence of residual or disseminated malignancy and indicate poor prognosis. Useful for both identifying hormone-dependent tumors and as prognostic markers. Identifies those patients who will respond to hormone therapy and those who will need more aggressive treatment. If ER and PR are both positive, there should be a better response to hormone therapy. ER-positive tumors have been Cytosol from breast associated with longer disease-free interval for the patient and tumor tissue possibly longer survival. Estrogen and Progesterone Receptors (ER and PR) Human Chorionic A glycoprotein hormone consisting of an - and -chain. Gonadotropin Increased in pregnancy with peak between 8th and 12th week of (hCG) gestation. Increased in trophoblastic cancers such as choriocarcinoma and hydatidiform mole. AFP is normal in these Serum or urine forms of cancer. hCG (and free) and AFP are increased in nonseminomatous germ-cell tumors of the testes and are essential for monitoring clinical management. 180 TEST & SOURCE CLINICAL USE Elevated in prostatic cancer. Useful for monitoring treatment of Prostatic Acid Phosphatase (PAP) patients with confirmed prostatic cancer. Elevated levels following treatment may indicate residual or recurrent disease. Test has Serum relatively low sensitivity. Prostate-Specific Antigen (PSA) Serum SOURCE: A glycoprotein and serine protease. Increased in prostatic cancer but also in benign prostatic hypertrophy (BPH). Useful in monitoring treatment of patients with prostate cancer. Increasing the cut-off point to 10 g/L reduces positive results from BPH to <2-3%. PSA exists in the free form and bound to proteins like 1antichymotrypsin. Measurement of the ratio of free to total PSA may have advantages in the differential diagnosis of BPH, especially in the diagnostic “grey level” of 4-25 g/L of total PSA. The proportion of free non-complexed PSA to total PSA is significantly lower in patients with untreated prostate cancer than in patients with BPH. PSA Probability of Cancer 0-2 ng/mL 1% % free PSA Probability of Cancer 2-4 ng/mL 15% 0-10% 56% 4-10 ng/mL 25% 10-15% 28% 10 ng/mL 50% 15-20% 20% 20-25% 16% American Medical Association Family Medical Guide, 4th ed., Hoboken, NJ: Jon Wiley & Sons, 2004. Her2/neu Her2/neu is human epidermal growth factor receptor. Her2/neu is used to predict response of breast tumors in chemotherapy. Herceptin (trastuzumab) is a humanized monoclonal antibody that binds to Her2/neu and inhibits activation of the receptor, consequently breast tissue that is positive for Her2/neu are candidates for Herceptin therapy. Currently the test is best performed on breast tissue biopsy specimens. 181 E. CASE STUDY CASE 1 (J. Urol. 170(4):1305, 2003) A 59-year-old man underwent radical prostatectomy for prostate adenocarcinoma. Serum PSA at diagnosis was 8.5 ng/ml (Hybritech assay). Pathological examination of the removed tissue showed evidence of extracapsular extension, positive margins and bilateral seminal vesicle involvement. Following surgery the patient received adjuvant radiation therapy to the prostate bed. Postoperatively, PSA was undetectable (Hybritech assay). Ten months following surgery, PSA measured 37 ng/ml on the AxSYm assay (normal range <4 ng/ml). Repeat AxSYm assay PSA measurement 2 weeks later was 31.3 ng/ml. Evaluation, including bone scan and abdominal computerized tomography, was negative for metastatic disease. Therapy was initiated with 10.8 mg goserelin depot injection and 50 mg bicalutamide daily. Three weeks after initiation of hormonal therapy PSA measured 0.02 ng/ml by Hybritech but 6 weeks later measured 28.2 ng/ml by AxSYm. Because of this discrepancy, repeat PSA’s were measured within 3 days of each other using the different assays, and results varied significantly with the PSA measuring less than 0.01 ng/ml on the Hybritech assay but 23.6 ng/ml on the AxSYm assay. 1. Where the tumor marker assays used appropriately in this case? 2. Describe the mechanism of action of goserelin and bicalutamide. 3. What other lab test might have been useful prior to prescribing goserelin and bicalutamide? 4. Give a possible reason for the discrepancy in the PSA results. 182 XIV PREGNANCY & PRENATAL ANALYSIS 183 184 A. OBJECTIVES To understand the differences between serum and urine pregnancy tests To describe how “over-the-counter” pregnancy tests work To introduce laboratory examination of amniotic fluid B. KEY TERMS Ectopic pregnancy - occurs when blastocyst implants in a location other than the uterus Hydatidaform mole - abnormal pregnancy resulting from pathologic ovum C. BACKGROUND/SIGNIFICANCE It is advantageous to diagnose a pregnancy as promptly as possible. The diagnosis of pregnancy is initially made by measuring human chorionic gonadotropin (hCG) in urine or blood. Since this test is available over the counter in most pharmacies and since positive results can result from causes other than a viable pregnancy it is important that pharmacists understand how to interpret these tests. D. DIAGNOSIS OF PREGNANCY The tests used in the clinical laboratory for the diagnosis of pregnancy are based on immunological techniques to detect the presence of hCG in urine or blood. hCG is a glycoprotein of 40,000 MW consisting of an alpha and a beta subunit. The alpha subunits of luteinizing hormone (LH), follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH) and hCG are identical while their beta subunits are unique for each hormone and are responsible for their biological action. Antibodies formed against the whole hCG molecule cross-react extensively with LH, FSH, TSH and this has been a source of bothersome interference with the specificity of pregnancy tests. A major improvement occurred with the development of specific antibodies against the beta subunit of hCG, such that the test is not affected, for instance, by the mid-cycle or menopause elevations of LH. Unfortunately the hopes for complete specificity of this test have not been borne out in practice. Traces of hCG have been demonstrated in the blood of normal, healthy men and nonpregnant females. The sources of this hCG appear to be the testes and the pituitary. Furthermore, anti-hCG-betasubunit antibodies do cross-react to a small extent with other hormones, particularly with LH. Thus, values below 5 mIU/mL of serum are interpreted as negative and values over 30 mIU/mL as positive. With the most sensitive methods, values indicating pregnancy are usually reached after the first week post-conception; i.e., well before the first missed menstrual period. Quantitation of hCG in blood also is used in the diagnosis and monitoring of therapeutic response in trophoblastic neoplasms and non-seminomatous germ-cell tumors. 185 The serum values related to pregnancy are: Nonpregnant 0-5 mIU/mL Indeterminate 6-20 mIU/mL First week post-conception 20-30 mIU/mL Second week post-conception 30-100 mIU/mL Third week post-conception 100-1000 mIU/mL First trimester peak (7-12 weeks) 10,000-160,000 mIU/mL Second trimester 6,000-30,000 mIU/mL Third trimester 400-15,000 mIU/mL The measurement of -hCG in serum is also useful in the diagnosis of ectopic pregnancy which may produce an “acute abdomen.” In this situation the measurement is extraordinarily important in the workup of the etiology of the abdominal pain and is carried out with the quantitative serum test. If positive, life saving surgical intervention may be indicated. In ectopic pregnancy, -hCG decreases in serum over time. PRINCIPALLY, THREE ANALYSES ARE NOW USED: 1. URINE HOME PREGNANCY TEST KITS Drugstores now carry urine pregnancy testing products that are easy to use, 99% specific and sensitive (when used as directed) and relatively inexpensive. The detection limit for pregnancy is the day of the first missed period. If negative they recommend retesting in 5-7 days if menses has not occurred. Specimens collected from pregnant women less than twelve days from conception may contain concentrations of hCG below the limit of detection of the test. In these instances the test should be repeated two to three days later since hCG approximately doubles every two days during early pregnancy. This allows the increase in concentration of hCG to reach the detection limit of the assay. 2. URINE QUALITATIVE PREGNANCY TEST - CLINICAL LABORATORY The rapid and sensitive qualitative Color Immunochromatographic Assay (Quidel, San Diego, CA) utilizing a monoclonal/polyclonal antibody method capable of detecting the intact molecule of hCG in urine is used at both the UCSD and VA Medical Centers. This test unit consists of a plastic container with a membrane strip which provides the solid support for the immunochromatographic assay. One end of the membrane provides contact with the urine well, which contains an absorbent pad which provides an even flow of the urine along the membrane. In the test, 220 L of urine is added to the “Add Urine” well, saturating the absorbent pad which then transports the urine to the attached membrane strip. As the urine moves to the first zone of the membrane, it mobilizes the mouse anti--hCG monoclonal antibody conjugated with red latex beads. The urine continues to move the antibody-red latex conjugate across the membrane to the immobilized anti--hCG zone, a rabbit polyclonal antibody which is immobilized on the vertical line. If hCG is present in the urine specimen, a “sandwich” of solid phase/hCG/redtex is formed. The vertical line will appear, resulting in a positive sign (+) visible in the “Read Results” window, indicating the presence of hCG. If hCG is not 186 detected, the “Read Results” window will only contain the pre-printed blue horizontal line, indicating a negative (-) result. As urine continues to move across the membrane, it comes in contact with the reagent in the “Test Complete” window. A blue line will appear, indicating the test is complete. See Figure. Sensitivity: 30 mIU/mL In normal pregnancy, hCG concentrations can reach this level as early as 7 to 10 days post conception. Specimen For optimal results it is best to test the first urine voided in the morning, because it contains the greatest concentration of hCG. However, urine collected anytime during the day can also be used. Limitations 1. Positive results from very early pregnancy may later prove negative due to natural termination of pregnancy. This is estimated to occur in up to 50% of all conceptions. It is recommended that weak positive results be retested with a fresh urine sample 48 hours later. 2. A negative result obtained with a urine specimen collected from a very early pregnancy (or a very dilute urine) should be retested on a fresh specimen after two days. 3. Patients with trophoblastic and nontrophoblastic disease may have elevated hCG levels; therefore, the possibility of hCG-secreting neoplasms should be ruled out prior to the diagnosis of pregnancy 3. SERUM QUANTITATIVE PREGNANCY TEST - CLINICAL LABORATORY Serum should be used in the quantitative -hCG assays. The assay range is from 1.9 mIU/mL to 500 mIU/mL and may be extended by dilution of the specimen by the laboratory. This detection limit enables the laboratory to detect pregnancy about 2 days before the urine qualitative method gives a positive result. This test should be used in the detection of ectopic pregnancy and tumors. 187 E. DIAGNOSIS AND MONITORING OF ERYTHROBLASTOSIS FETALIS: AMNIOTIC FLUID ABSORBANCE In normal pregnancy a small concentration of unconjugated (indirect) bilirubin is present in the amniotic fluid, and this concentration falls as term is approached. In erythroblastosis fetalis (e.g., due to Rh-incompatibility), however, the bilirubin concentration increases with time as the hemoglobin liberated by the hemolytic process is converted to bilirubin. Due to fetal hepatic immaturity all bilirubin is generally of the unconjugated type and is bound to albumin. When the bilirubin concentration is so high that the binding capacity is exceeded, the free unconjugated bilirubin can penetrate the brain and produce kernicterus. Hence, the assessment of the presence and severity of erythroblastosis fetalis relies heavily on the monitoring of bilirubin in the amniotic fluid. Sensitization of pregnant women is due to exposure to Rh-positive fetal blood which can gain access to the maternal circulation. Although this antigenic challenge is generally small, it may be sufficient in some women to provoke an antibody response. A much larger antigenic exposure may result from disruption of the integrity of the fetal compartment during spontaneous or induced abortion, ectopic pregnancy, or delivery. When a sensitized woman has another Rh-positive pregnancy, the antibodies (usually of the IgG class) can readily cross the placenta and can cause destruction of fetal erythrocytes leading to production of bilirubin. Repeated exposure during pregnancy or delivery leads to an augmented response and to more severely affected later pregnancies. Sensitization can usually be prevented by intramuscular administration of anti-Rho (D) immune globulin (RhIg, RhoGAM, Ortho Diagnostic systems) which inactivates the potentially stimulating immunogens. A small number of sensitized pregnancies, however, continues to occur despite immunization, and in pregnancies in which immunization has not been performed. DETERMINATION OF BILIRUBIN Although bilirubin in serum is measured colorimetrically by diazotization methods, bilirubin in amniotic fluid is monitored by direct visible scanning spectrophotometry of a centrifuged specimen. Direct spectrophotometry is utilized for the following reasons: 1. It is much more rapid than “wet” chemical methods; 2. The presence of substances other than bilirubin (e.g., hemoglobin) can be inferred from the shape of the visible spectrum (Figure 1). 3. There is analytical interference in chemical methods by amniotic fluid Bilirubin in serum is not determined by scanning spectrophotometry due to the presence of other interfering compounds in serum. The bilirubin content obtained by visible spectrophotometry is expressed as absorbance. The absorbance (formerly optical density or “O.D.”) of bilirubin at its wavelength of maximum absorption (450 nm) is measured and corrected for background absorbance by 188 subtraction of a constructed tangent baseline (Figure 1). Hence the term “delta A” (or “delta O.D.”). Bilirubin is highly photosensitive, and this is particularly true in amniotic fluid, in which concentrations are generally low and in which few cells are present to protect the bilirubin from light. Hence amniotic fluid for bilirubin measurement must be protected from light by collecting it into an amber-colored tube or (preferably) by wrapping the tube with aluminum foil or masking tape. Frequently hemoglobin may be present in amniotic fluid concurrently with bilirubin due to the ongoing hemolytic process (hemoglobin of fetal origin) and/or to repeated amniocentesis (hemoglobin of maternal origin). Since the so-called “Soret band” of hemoglobin (410 nm) is near the absorbance band of bilirubin (450 nm) (Figure 1), substantial positive interference can occur in the determination of absorbance. Therefore, whenever hemoglobin is noted in the initial scan of the fluid, the fluid is extracted with an equal volume of chloroform. Since the bilirubin is highly soluble (100% recovery) in chloroform and hemoglobin is insoluble in chloroform, the chloroform extract will contain only bilirubin. Since a volume of chloroform equal to that of the amniotic fluid is used, the absorbance of bilirubin in the chloroform extract will be equal to that in the original amniotic fluid, free from interference by hemoglobin (Figure 1). Amniotic fluid absorbance values are interpreted by plotting them semilogarithmically on a “Liley-Prognostication Chart”, which contains weeks of gestation on the abscissa (linear axis) and absorbance on the ordinate (logarithmic axis). The chart is divided into three zones: “zone C - very severely affected babies” (zone of severe hemolysis), “zone B - indeterminate zone,” and “zone A - unaffected babies” (no hemolysis). Persistence of values in the severely affected zone may induce the clinician to perform intrauterine transfusion or (if the fetus is determined to be viable by the tests previously described) to induce labor. Frequently exchange transfusion is performed at birth in such situations. A plot of serial amniotic fluid absorbance is shown in Figure 2. DIAGNOSIS OF GENETIC ABNORMALITIES Sufficient cells can be recovered from amniotic fluid to permit culture and subsequent karyotyping for such abnormalities as Down syndrome (trisomy 21). In addition, sex of the fetus can be determined (important in certain X-linked disorders when one or both parents are affected). 189 Figure 1: Determination of Amniotic Fluid Absorbance (30 weeks gestation) Upper = Visible spectrum of unextracted amniotic fluid (Note the absorption band of bilirubin at 450 nm and the interfering Soret band of hemoglobin at 410 nm) Lower = Visible spectrum of chloroform extract of same amniotic fluid (Note the single absorption band of bilirubin at 450 nm, free from hemoglobin interference) Absorbance = 0.11 (“indeterminate zone” of Liley chart) 190 Figure 2: Liley Amniotic Fluid Prognostication Chart 191 G. CASE STUDY CASE 1 (BrighamRAD Teaching Case Database <www.brighamrad.harvard.edu/Cases /bwh/hcache/38/full.html>, August 19, 1994) An 18-year-old woman at 11.5 weeks gestation presented with an elevated serum betasubunit human chorionic gonadotropin (b-hCG) concentration of 285,730 mIU/ml. Transabdominal ultrasound images show complex solid and cystic areas filling the endometrial cavity of the uterus. 1. What is the differential diagnosis in this case? 2. What drugs should be used to treat this patient? 192 XV LABORATORY DIAGNOSIS OF IRON AND RED BLOOD CELL DISORDERS 193 194 A. OBJECTIVES To describe components of basic hemogram To list different laboratory tests for iron deficiency and iron excess To describe iron-deficient anemia, megaloblastic anemias, sickle cell anemia and anemia of chronic disease B. KEY TERMS Anemia - reduction in red cell mass Apotransferrin - plasma iron binding protein with no iron bound Ferritin - a serum protein that is used to help determine iron status, a chief storage form of iron in other tissues Folic acid - essential cofactor for one carbon transfers, DNA synthesis Hemoglobin S disease - homozygous state in which patients only express sickle beta chains, clinically and hematologically abnormal Hemoglobin S trait - heterozygous state in which there is one normal beta chain and one sickle beta chain, no clinical or hematological manifestations Hgb - hemoglobin (g/dL) - predominate adult form (hemoglobin A) has two alpha chains and two beta chains that form a tetramer Hct - hematocrit (%) - a measure of the percent red blood cells relative to plasma volume Iron - measurement of the total amount of iron in serum MCH - mean corpuscular hemoglobin in picograms (pg) MCHC - mean corpuscular hemoglobin concentration (g/dL) MCV - mean corpuscular volume in femtoliters (fL) MPV - mean platelet volume RBC - red blood count in a cubic millimeter reported as number of cells per L (106/L) RDW - red cell distribution width, a measure of the variation of cell volume Reticulocyte - a young red blood cell TIBC - total iron binding capacity, determined by amount of transferrin Thalassemia - decreased production of one or more hemoglobin chains 195 Transferrin - plasma iron binding protein with iron bound Vitamin B12 - essential cofactor for synthesis of folates and other biomolecules; major source of B12 is from animal proteins, cannot be synthesized by humans; stored in liver C. BACKGROUND SIGNIFICANCE Anemia can be caused by a variety of mechanisms and proper treatment depends on successful identification of the etiology of the anemia. A complete blood count (CBC) provides essential information for the diagnosis and treatment of a variety of hematological disorders. Consequently a basic understanding of the hemogram is important for diagnostic and treatment purposes. Iron, B12, and folate status are also important in hematology and a variety of disease states. The laboratory plays a key role in the diagnosis and treatment of both B12 and iron deficiency as well as iron-overload states. D. COMPLETE BLOOD COUNT (CBC) 1. CBC INCLUDES THE FOLLOWING: Red cell parameters: RBC, Hgb, Hct, MCV, MCH, MCHC, RDW Leukocyte (WBC) parameters, at minimum WBC count; a differential leukocyte count also quantifies the neutrophils, lymphocytes, monocytes, eosinophils, and basophils Platelet parameters: count and Mean Platelet Volume (MPV) Subjective assessment of cell morphology by examination of peripheral blood smear (e.g., shape, size, and color intensity of red cells, as well as granularity and segmentation of white cells) Table 1: CBC Results in Various Conditions Parameter RBC Hgb Hct MCV MCH Anemia ↓ ↓ ↓ ↓ (iron deficiency) ↓ Thalassemia AS SS trait trait disease ↑ − ↓ − − ↓ − − ↓ ↓ ↓ − − ↑ ↑ 2. AUTOIMMUNE HEMOLYTIC ANEMIA Autoimmune antibodies develop against red blood cell antigens causing red cell death (hemolysis) Variety of causes, can be drug-induced (quinine, quinidine, and fluoroquinolone antibiotics) Results in hemolysis 196 Table 2: Interpreting CBC Results E. ASSESSMENT OF IRON DEFICIENCY AND IRON OVERLOAD 1. IRON DEFICIENCY The incidence of iron deficiency is about 9% in toddlers, 2% in men, 11% in menstruating women, and nearly 50% in pregnant women. The cause in children is frequently dietary deficiency; e.g., milk has a very low content of iron. In adults, the cause is generally chronic blood loss. 2. CONDITIONS OF IRON OVERLOAD The incidence of iron overload in the US is 1%. There are three main conditions of iron overload: Hemosiderosis implies iron overload without tissue injury. Hemochromatosis is a condition of iron overload with associated tissue injury. It is usually caused by chronic, excessive absorption of iron. 197 Hereditary hemochromatosis is the classic disorder of iron overload. The prevalence of homozygosity is about 5 per 1,000. In all three conditions, serum iron, iron saturation of transferrin, and ferritin are high. Transferrin an d TIBC are usually low in hemochromatosis. Apo transferrin UIBC TIBC: 250-440 g/dL Serum Iron (III) (bound to transferrin) M: 65-170 g/dL F: 50-170 g/dL Saturation: M: 20-50% F: 15-50% of TIBC Transferrin M: 187-318 mg/dL F: 218-331 mg/dL UIBC = Unsaturated Iron Binding Capacity TIBC = Total Iron Binding Capacity 3. SERUM IRON Serum iron is defined as Fe (III) bound to transferrin and to a much lesser extent to some other serum proteins. Iron bound to free hemoglobin is not included. Decreases in serum iron are seen in iron deficiency and in chronic inflammatory disorders. Serum iron may also be decreased in the early stages of treatment for anemias, e.g. the treatment of pernicious anemia with vitamin B12. In this case, hemoglobin synthesis draws iron from serum and iron stores. Other causes of low serum iron are hemorrhage, blood donation, and menstruation. Increases in serum iron are seen after use of oral contraceptives (increased synthesis of transferrin), in iron overload conditions such as hemosiderosis and hemochromatosis, acute iron poisoning, ingestion of iron medication (300 mg tablet of FeSO 4 raises serum iron by 300-500 g/dL), parenteral iron administration, and hepatitis (release of iron from liver iron stores). 4. SERUM TOTAL IRON BINDING CAPACITY (TIBC) Definition: measurement of the maximum amount of iron that serum proteins, mainly transferrin, can bind. The TIBC is increased in iron deficiency. The combination of low serum iron and increased TIBC is helpful in the diagnosis of iron deficiency. TIBC and transferrin are decreased in acute inflammation (transferrin = negative acute phase reactant), in chronic inflammatory disorders (decreased transferrin synthesis), in malignancies, and in malnutrition. 198 The percent saturation of transferrin is an additional diagnostic tool. Normal values are 20-50% in males, and 15-50% in females. It is low in iron deficiency and high in hemochromatosis. In the latter condition, total transferrin and TIBC are frequently low. The portion of transferrin that is not saturated with iron is called apotransferrin or unsaturated iron binding capacity (UIBC). 5. SERUM FERRITIN Ferritin is the chief iron storage form in the body. Serum ferritin is very low (about 1% of total serum iron) and is in equilibrium with the body iron stores. In ferritin, iron is present as micelles of hydrated ferric oxide-phosphate complexes, adhering to the inner surface. Ferritin is a very sensitive indicator of iron status, especially for iron deficiency except in chronic inflammatory disorders like rheumatoid arthritis, renal disease, and malignancies (predominantly lymphomas, leukemia, breast cancer, and neuroblastoma) in which ferritin is increased. High serum ferritin values are also seen in viral hepatitis (release from iron stores) and in iron storage diseases. Reference Range: M: 30-400 ng/mL; F: 10-145 ng/mL 6. CYTOCHEMICAL STAINING Cytochemical staining of hemosiderin in bone marrow aspirates by the Prussian blue reaction is the most definitive test for iron status. It is the accepted “gold standard.” 7. QUANTITATIVE IRON IN LIVER Measurement of iron in liver biopsies is also very helpful in the diagnosis of hemochromatosis, especially in cases in which the serum iron and iron-binding capacity may be borderline abnormal. Normal: 100-300 ng/g wet tissue 199 F. CASE STUDIES CASE 1 (Ann. Pharmacother. 37(7):1010-1013, 2003) An 82-year-old man, weighing 54 kg and 168 cm in height, presented to the emergency department (ED) with symptoms of jaundice, fatigue, dizziness, loss of vision, and shortness of breath. Vital signs on admission revealed T 36.7°C, HR 66 beats/min, and BP 96/46 mmHg. The patient’s calculated creatinine clearance was 48 mL/min. His chronic medical conditions included hypertension and coronary artery disease. He had been prescribed a 10day course of levofloxacin (500 mg/day) for cellulitis subsequent to injuries to his leg. Approximately 4 days after levofloxacin initiation, the man noticed symptoms of fatigue and dizziness. By the end of the 10-day treatment with levofloxacin, the symptoms had progressed and included jaundice, severe fatigue, Coca-Cola-colored urine, loss of vision, and shortness of breath. On admission to the ED, blood work revealed hemoglobin 7.0 g/dL, hematocrit 19%, lactate dehydrogenase (LDH) 1543 IU/L, and unconjugated (indirect) bilirubin 4.8 mg/dL. Electrolytes and liver enzyme tests were within normal limits. 1. Propose a drug-induced cause for these observations 2. Is this type of adverse drug event common for this drug? This class of drugs? 3. How should this patient be treated? 4. What other laboratory tests might be useful? 200 CASE 2 Patient presents to clinic because he ran out of atenolol. PMH includes hypertension, hyperlipidemia, impaired fasting glucose, and microcytic anemia. Routine labs include TIBC 585 ug/dL (normal 228-428), transferrin 418 mg/dL (normal 200-400), Fe 11 ug/dL, ferritin 5 ng/mL (normal 30-400). 1. What is this patient’s % iron binding saturation? Is it consistent with the rest of his clinical picture? 2. How would you classify this patient’s iron status? 3. What kinds of drugs could cause these findings? 4. What pathological conditions could cause these findings? 5. What other laboratory tests might be indicated? 201 202 XVI HEMOSTASIS AND WHITE BLOOD CELL DISORDERS 203 204 A. OBJECTIVES To describe the elements required to form a platelet plug To describe the coagulation cascade To understand the differences between prothrombin time, partial thromboplastin time and international normalized ratios To understand how to interpret white blood cell count in inflammation and leukemia B. KEY TERMS Aplastic anemia - diverse group of bone marrow disorders characterized by pancytopenia due to reduction of hematopoetic marrow stem cells B cell lymphocyte - responsible for humoral immunity, makes plasma cells that produce antibodies Basophil - readily stained with basic dyes, leukocyte with pale staining nucleus usually consisting of two lobes and prominent blue granules containing vasoactive amines such as histamine and serotonin Coagulation - enzyme cascade to form a fibrin clot Eosinophil - a leukocyte with prominent red granules and a nucleus with two lobes, readily stained with eosin Granular leukocytes - leukocytes with abundant granules in cytoplasm (neutrophils, eosinophils and basophils) Hemostasis - the process of stopping bleeding Hypoplastic anemia - general term indicating a form of anemia due to varying degrees of erythrocytic hypoplasia Leukemia - progressive malignant disease of the blood - forming organs, characterized by distorted proliferation of leukocytes and their precursors Leukocyte - white blood cell Leukopenia - reduction in number of leukocytes in blood (< 5000/mm3) Lymphocyte - immunologically competent cells, divided into T and B cell lines Lymphoma - malignant disease of lymphocytes, producing enlarged lymph nodes and spleen Lymphopenia - reduction in number of lymphocytes Monocyte - a mononuclear phagocytic leukocyte Neutropenia - a decrease in the number of neutrophilic leukocytes in blood 205 Neutrophil (polymorphonuclear leukocyte, segmented leukocyte) - a granular leukocyte having a nucleus with 3 to 5 lobes, having properties of chemotaxis, adhering to immune complexes, and phagocytosis; these cells are the most important in defense against bacterial infection Nongranular leukocytes - leukocytes without granules in cytoplasm (lymphocytes and monocytes) Pancytopenia - deficiency of all cellular components of blood Reticulocyte - a young red blood cell showing basophilic reticulum (rough endoplasmic reticulum on which hemoglobin is synthesized) T cell lymphocyte - responsible for cellular immunity, T helper, cytotoxic and suppressor cells Thromobocyte - a blood platelet Thrombocytopenia - reduction in number of platelets von Willebrand’s disease - autosomal dominant hemorrhagic disease caused by lack von Willebrand factor (vWF) and low coagulation factor VIII which is carried on vWF C. BACKGROUND SIGNIFICANCE Hemostasis is essential for life and is tightly controlled through complex processes including formation of a platelet plug, activation of the coagulation cascade and through vasoconstriction. Many diseases and drugs affect hemostasis and the hematology laboratory provides key information to diagnose, treat and monitor coagulopathies. In addition to hemostasis, the hematology laboratory also provides quantitation of a variety of white cells and precursors essential for detecting disease and/or drug-induced changes to leukocytes. D. HEMOSTASIS 1. PLATELET PLUG (SEE FIGURE 1) A) With tissue injury, platelets adhere to vWF through GPIb-IX-V, vWF adheres to subendothelial matrix. B) Platelets adhere to collagen through GPIa-IIa. C) Platelets aggregate when GPIIb-IIIa binds to fibrinogen after platelets are activated (thrombin, ADP, collagen are activators). D) Activated platelets secrete ADP, and thromboxane (which causes vasoconstriction). Activated platelets synthesize and display factor Va on their surfaces. 206 I II n fibri IIa a VaX l Plate IIb IIIa Platelet fibrinogen (I) IIIa IIb l Plate Ia IaI -V I- X Ib P G WF v et l Plate et et endothelial cell collagen Figure 1: Formation of a Platelet Plug 2. COAGULATION CASCADE A sequence of proteolytic cleavages of preformed enzymes that eventually leads to thrombin cleaving peptides from fibrinogen, forming fibrin which polymerizes. A) Platelet plug initiates and is required for coagulation cascade B) Form a fibrin clot 1) Injured endothelium makes tissue factor (TF) 2) TF clips and activates circulating factor VII 3) Factor VIIa activates circulating X and IX 4) Xa binds to Va on platelets, localizing enzyme complex that converts II (prothrombin) to IIa (thrombin) 5) IIa converts fibrinogen to fibrin which polymerizes to form a clot 6) XIII crosslinks fibrin to form stable clot C) Backup and amplifying systems 1) IXa complexes with VIII carried on vWF to form IXaVIIIa 2) IIa activates more VIIIa and Va, XIa 3) Collagen activates circulating XII into XIIa which activates XI into XIa which upregulates IX into IXa 4) IIa also activates XI into XIa 207 D) Moderating factors that localize clot 1) Thrombomodulin on endothelial cells, changes the activity of factor II causing it to activate protein C. Protein C combined with S inactivate V and VIII. 2) Antithrombin III, a circulating molecule is activated by cell surface heparan (same molecule as circulating heparin). When heparan is activated it inhibits factors II and X. 3) Plasmin, which is activated by the factor XII complex, digests fibrin clots eventually releasing d-dimer. 3. PROTHROMBIN TIME (PT) Measured in seconds (normal approximately 10 seconds) Time for conversion of prothrombin to thrombin Evaluates the factor VII dependent pathways of the coagulation cascade Performed by adding tissue factor (TF) to plasma TF (+ platelet phospholipids, Va) → VIIa → Xa Va → IIa → I → clot Often converted into International Normalized Ratio (INR) which is (patient PT/Control PT)ISI where the ISI is the international sensitivity index (a measure of the reagents sensitivity to VIIa); normal INR is 0.9-1.5 PT’s are sometimes difficult to compare between laboratories, INR can be directly compared Best for monitoring coumadin therapy (which primarily inhibits synthesis of VII as well as II, IX and X) Patients on anticoagulant drugs should have an INR of 2.0 to 3.0 for basic “bloodthinning” needs For some patients who have a high risk of clot formation, the INR needs to be higher (2.5 to 3.5) 4. PARTIAL THROMBOPLASTIN TIME (PTT ALSO APTT) Measured in seconds (normal is 23 to 33.5) Time for clot to form Evaluates the factor XII dependent pathways Performed by exposing blood to negatively charged surface Negative surface → XIIa → XIa → IXa, VIIIa → Xa Va → IIa → I → clot Best for monitoring heparin which mainly inhibits II; it also inhibits X, XI, and XII because IIa amplifies these factors PTT is set screen for hemophillia A and B 5. OTHER LAB TESTS Don’t analyze for all cofactors Mix patient sample with commercially available plasma that is devoid of specific cofactors and see if clotting occurs; if coagulation does not occur then can isolate missing cofactor 208 collagen vWF Heparan ATIII Inhibits IIa, Xa Thrombomodulin IIa Platelet VII + TF VIIa IXa vWF XaVa Va VIIIa } Platelets Va XIII XIa II IIa I C Ca + Sa (inactivates Va + VIIIa) Crosslinked Fibrin Clot D^D etc. plasmin XII - surface D dimer Figure 2: Coagulation Cascade 6. ANTICOAGULANT DRUGS Heparin Requires antithrombin III (ATIII) for anticoagulant effects With ATIII inhibits thrombin (Factor II), IXa and Xa Uses include treatment for: venous thrombosis, pulmonary embolism, initial management of unstable angina and acute myocardial infarction, coronary angioplasty and cardiac bypass surgery Warfarin Antagonist of Vit K Inhibits carboxylation of factors II, VII, IX and X, thus preventing these coagulation factors from becoming active No effect on circulating, active forms of the above coagulation factors, therefore delayed onset of action Also inhibits synthesis of factors C and S so may paradoxically enhance clotting in some patients 209 7. THROMBOLYTIC DRUGS Streptokinase Forms non-covalent attachment with plasminogen activating it to form free plasmin Free plasmin digests fibrin clots Tissue Plasminogen Activator (t-PA) Activates plasminogen bound to fibrin Reteplase is a deletion mutant of t-PA 8. ANTIPLATELET DRUGS Aspirin Acetylates serine residues on cyclooxygenase Prevents formation of thromboxane A2 Thromboxane A2 causes vasoconstriction and platelet aggregation Clopidogrel (Plavix) Inhibits platelet aggregation by blocking purine receptor May cause thrombotic thrombocytopenic purpura (TTP) Abciximab (Reopro) Fab fragment of humanized monoclonal antibody directed against IIb/IIIa receptor 9. PLATELETS ↑ in inflammation, disseminated cancer ↓ idiopathic thrombocytopenic purpura (ITP), thrombotic thrombocytopenic purpura (TTP), bone marrow failure ↓ in disseminated intravascular coagulation (DIC) E. WHITE CELLS WBC (Leukocytes) Lymphocytes B Cells Antibody production T Cells T helper T cytotoxic Granulocytes Neutrophils Phagocytic against bacteria coated with Ab Receptors specific for bacteria Kill with lysosomes Eosinophils Attracted to the site of allergy IgE receptors Granules with allergic response (not histamine) Figure 3: White Blood Cells 210 Basophils Contain vasoactive amines, histamine and serotonin Monocytes Phagocytic and present bacterial antigen to T helper cells 1. WHITE BLOOD CELLS ↑ in infection and inflammation ↑, −, or ↓ in leukemia Key difference is that leukemia has immature cells ↓ in aplastic anemia (often drug-induced), often do not recover ↓ in hypoplastic anemia, often recover after drug stopped 2. NEUTROPHILS ↑ with steroid administration ↑ with inflammation (bacterial infection) Variable effect with leukemia, sometimes greatly ↑, particularly immature cells 3. LYMPHOCYTES ↑ with viral infections ↓ with steroid administration, immune deficiency 5. NEUTROPENIA, LYMPHOPENIA, AND THROMBOCYTOPENIA ARE OFTEN DRUG-INDUCED Not always cytotoxic chemotherapy Immune mediated or idiosyncratic Stop drug One cell line decreased usually indicates drug-induced vs. all cells decreased usually indicates a stem cell problem (disease or drug-induced) 211 F. CASE STUDIES CASE 1 (Ann. Pharmacother. 37(2):212-215, 2003) A 71-year-old woman with numerous medical conditions, including type 2 diabetes mellitus, atrial fibrillation, hypothyroidism, congestive heart failure, and gastroesophageal reflux disease, was enrolled in an anticoagulation clinic where telephone follow-up of prothrombin time/international normalized ratio (INR) is performed. She was anticoagulated with warfarin (24 mg/wk) for atrial fibrillation and had a stable INR of 2.1. Several weeks after starting fenofibrate (200 mg/d) her INR was noted to be 6.7 during routine monitoring. Upon telephone interview, her caretaker denied that the patient had recent alcohol intake and any changes in medicines, health status, or diet. 1. How do you interpret the change in this patient’s INR? 2. What other laboratory tests might be helpful in the management of this patient? 3. What dose recommendations would you suggest? 4. Propose a mechanism for this interaction. 5. Are interactions common with these kinds of drugs? 212 CASE 2 (Therapeutics & Toxins News 18(1):1-3, 8-10, February 2003) A 19-year-old 70 kg healthy male presented to ED with concerns he had been poisoned at a restaurant with pellets that resembled d-Con Mouse Prufe-IIO. A computer search indicates that the active ingredient is brodifacoum. Lab results include a PT of 11.3 and an INR of 1.2. The patient is discharged without treatment. Two days post exposure the patient again presents to a different ED complaining of stomach pain and blood in his stool. Lab results include a PT of 12.5 and an INR of 1.2. Again the patient is discharged without treatment. Eight days post exposure the patient presented to a clinic complaining of abdominal discomfort, dizziness, bloody stool and nosebleeds. Lab results include a PT 94.7, INR 9.2, and Hct 42.2%. 1. How do you explain these symptoms and laboratory findings? 2. Why did it take days before the PT and INR became elevated? 3. Why is the Hct not low? 4. What is the mechanism of action of this rodent poison? 5. What is the appropriate therapy for this patient? 6. How long will this patient need to be treated? 7. Is this a common type of poisoning? 213 214 XVII CLINICAL TOXICOLOGY 215 216 A. OBJECTIVES To understand the role of the laboratory in the diagnosis and treatment of toxicology cases To review the pathophysiology of frequently encountered toxic exposures To review basic pharmacokinetic parameters To be able to recognize specific drug overdoses B. KEY TERMS Clinical toxicology - the measurement and interpretation of concentrations of drugs and other toxic substances in human biological fluids for the purpose of patient care Drug abuse screening - the identification of “street” drugs (e.g., opiates, phencyclidine, amphetamines) in urine of subjects suspected of abusing these compounds Emergency toxicology - the laboratory diagnosis of the presence and severity of drug overdose, often in the comatose or obtunded patient Forensic toxicology - the measurement of drugs and toxins in tissues for medicolegal purposes (e.g., determining cause of death) Therapeutic drug monitoring (TDM) - the determination of whether plasma drug levels are therapeutic, subtherapeutic, or toxic so that dosages may be adjusted accordingly Toxicology - the analysis and study of harmful compounds in biological materials. C. BACKGROUND/SIGNIFICANCE There are over 2 million human exposures reported to poison control centers each year and 25% of these cases are seen in a health care setting. Many poisonings are accidental and the majority involve children. Most cases of poisoning require supportive therapy only, but in select cases effective antidotes are available and can be life-saving. Depending on the clinical setting, pharmacists often play a key role in treating patients after a toxic exposure. SAMPLE TYPES TO SUBMIT FOR THE OVERDOSED PATIENT Blood (whole blood or serum depending on analyte) is preferred whenever drug concentrations can be correlated with effects; quantitative analysis allows for interpretation of degree of toxicity for some compounds Urine specimens are preferred when the concentration in blood is too low for detection due to extensive metabolism and clearance of the drug (e.g., many drugs of abuse); for most compounds only qualitative analysis is performed Gastric fluid (available from lavage or emesis) is most useful for identifying the parent drug in overdose cases or if other fluids are not available or are not diagnostic; qualitative analysis often identifies parent drug Pills, capsules, tablets, powders found with the patient 217 In overdose cases it is important to share with the toxicology laboratory the clinical history in order to allow a time-saving “directed” drug search DRUG SCREEN There is no universal drug screen Important to communicate with the laboratory in order to understand what is covered in that particular lab’s “drug screen” One out of every four drug screens detects at least one drug unsuspected from the clinical history One out of every six drug screens detects only drugs unsuspected from the history To identify drugs for which specific treatment protocol exists (e.g., acetaminophen, digoxin) A “negative” toxicology report in overdose cases indicates the need to search for other causes of altered physical or mental status GENERAL TREATMENT OF DRUG OVERDOSE Comatose patients ABCD (Airway, Breathing, Circulation and Drugs) Supportive care alone (emesis, lavage, intravenous fluids, activated charcoal, cathartics, pressor agents; cardiac monitoring) Antidote if available and indicated Rarely: peritoneal dialysis, hemodialysis, or hemoperfusion D. PHARMACOKINETICS 1. DEFINITION: A mathematical description of the time course of a drug concentration in a patient Provides a framework for interpreting quantitative drug concentrations Figure 1 2. IMPORTANT CONCEPTS FOR TOXICOLOGISTS A) Zero-order kinetics: a constant amount of the drug is eliminated in a unit of time Good example is ethanol Can apply to drugs in an overdose situation when metabolic enzymes become saturated B) First-order kinetics (see Figure 1): a constant percent of the drug is eliminated in time Most drugs follow first-order kinetics C) Serum half-life: time required for serum concentrations to decrease by one half Only applies to first-order kinetics D) First-pass effect: applies to drugs which are cleared by the liver before reaching systemic circulation E) Steady-state: applies to repeated dosing and is reached in about 4 half-lives (94% of steady-state) 218 3. FACTORS WHICH CHANGE PHARMACOKINETICS A) Renal function Since many drugs are cleared by the kidneys it is often important to monitor creatinine clearance as an indicator of renal function B) Hepatic function Some drugs can induce liver enzymes (e.g., barbiturates) and decrease half-lives of other drugs Some drugs competitively inhibit metabolism of other drugs (cimetidine and coumarins) C) Saturation kinetics In an overdose situation primary metabolic routes become overloaded, half-lives no longer apply and excretion becomes zero-order Secondary metabolic routes can form toxic metabolites (e.g., acetaminophen) E. RECOGNITION OF SPECIFIC TOXIC EXPOSURES 1. ETHANOL Potent central nervous system depressant Most frequently encountered and often most significant drug in toxicology cases Effects vary with concentration; generally higher cortical functions (thought processes) are affected first followed by more basal functions (breathing) Common cause of hyperosmolality in emergency room patients Disulfiram (Antabuse) inhibits ALDH (Aldehyde dehydrogenase) causing a buildup of acetaldehyde which causes unpleasant effects (flushing, nausea, vomiting, etc.) Ethanol metabolism follows zero-order kinetics and for normal people is from 0.01 to 0.02% w/v/hr but can be induced to 0.03% w/v/hr Figure 2: Ethanol Three metabolic pathways of ethanol 1) Alcohol dehydrogenase in cytosol 2) Microsomal ethanol oxidation (inducible) 3) Peroxidase-catalase (minor) 219 2. METHANOL Toxicity is characterized by profound metabolic acidosis (uncouples oxidative phosphorylation and is metabolized to formic acid) Metabolites also cause blindness As little as 30 mL can be fatal Figure 3: Methanol Treatment Administer ethanol by drip until patients have ethanol concentrations of 100 to 150 mg/dL; ETOH competitively inhibits metabolism of methanol at ADH step Methanol can then be removed by dialysis (if > 50 mg/dL) Administer 4-methylpyrazole to inhibit alcohol dehydrogenase; hemodialysis may also be used 3. ISOPROPANOL Generally considered to have twice the CNS depressant effect as an equivalent dose of ethanol Metabolized to acetone Upper GI bleeding Belching (evaporates easily) NAD+ CH3CHOHCH3 Isopropanol NADH ADH Figure 4: Isopropanol 220 CH3COCH3 Acetone 4. ETHYLENE GLYCOL (PRINCIPLE ANTIFREEZE INGREDIENT) Same CNS depressant effects as ethanol but has toxic metabolites Progressively oxidized to glycolic acid and oxalic acid which causes myocardial depression and renal necrosis Serious toxicity at concentrations just detected by osmolal gap Hypocalcemia Figure 5: Ethylene Glycol Metabolism A) Three stages of ethylene glycol intoxication CNS depression (1-12 hours) Cardiotoxic (12-24 hours) Renal stage (24-72 hours) B) Treatment of ethylene glycol intoxication Ethanol to inhibit formation of toxic metabolites Bicarbonate for metabolic acidosis Ca++ replacement if necessary 4-methylpyrazole to inhibit alcohol dehydrogenase; hemodialysis may also be used 5. SALICYLATE (ASPIRIN) Analgesic, antipyretic and anti-inflammatory Toxicity characterized by metabolic acidosis (see Figure 6) Common symptoms include: tinnitus, hyperthermia, hyperventilation, CNS disturbances Acute overdoses are generally suicidal Chronic toxicity occurs at lower doses than acute overdose Treatment for salicylate overdose Hydration, glucose, K+ supplements, bicarbonate, hemodialysis 221 Stimulation of Medulla (350 mg/L) Metabolic Acidosis 1) Salicylates and other organic acids 2) Impaired renal function 3) Uncouple oxidative phosphorylation (increased organic acids) Hyperthermia and Respiratory Alkalosis Renal excretion of bicarbonate ACIDOSIS Respiratory Depression (Acidosis) Figure 6: Salicylate Acid-Base Disruption 6. ACETAMINOPHEN (TYLENOL) Analgesic and antipyretic, not anti-inflammatory Peak concentrations about 4 hours post-ingestion (for prediction of toxicity need to evaluate at this time, > 200 g/mL, hepatotoxicity) Normal half-life of 2-3 hours, if > 4 hours hepatic toxicity, if > l2 hours hepatic coma likely Single acute threshold for liver damage in adults is in the range of 150 to 250 mg/kg; children under age of 10 are more resistant to toxicity than adults Acetaminophen toxicity The primary route of metabolism of acetaminophen is glucuronidation or sulfation, with some also being metabolized to imidoquinone, a toxic intermediate. Normally the imidoquinone is inactivated by conjugation with glutathione. In an overdose situation all the glutathione is used up and the imidoquinone reacts with surrounding hepatocytes causing liver failure. N-acetylcysteine (Mucomyst) is the antidote and functions by supplying a substrate which replenishes glutathione stores. 222 Figure 7: Nomogram for Prediction of Acetaminophen Hepatoxicity MFO = mixed function oxidase Figure 8 223 A) Stages of acetaminophen toxicity which develop over several days GI distress (0 to 8 hours post dose): nausea, vomiting, malaise General well being (0 to 24 hours post ingestion) Liver toxicity (8 to 36 hours post ingestion): severe overdose leads to fulminant hepatic failure Recovery (days to weeks post dose): liver transaminases return to normal in 5-7 days, complete resolution takes weeks B) Treatment for acetaminophen overdose Activated charcoal (if patient presents early) N-acetylcysteine is the antidote Functions by binding the toxic metabolic intermediate N-acetylcysteine, if given early can completely prevent liver failure and death N-acetylcysteine administration is indicated in patients with acetaminophen concentrations above the nomogram treatment line, or with elevated AST, prolonged serum acetaminophen half-life, and history suggestive of significant acetaminophen overdose; patients with concentrations above the RumackMatthew line are at increased risk of hepatic toxicity 7. TRICYCLIC ANTIDEPRESSANTS Overdoses are monitored by EKG (better than blood concentrations, which do not correlate well with myocardial toxicity), prolonged QTc, widened QRS, arrhythmias Death due to cardiac arrest Effects include muscarinic blockade, block reuptake of norepinephrine, alpha adrenergic blockade, and membrane stabilization, all combine to give arrhythmias Selective Serotonin Reuptake Inhibitors (SSRI) have greatly decreased use of this class of drugs, but they are used if patients fail to respond to SSRI 8. BENZODIAZEPINES Anxiolytic/sedative/hypnotic depending on dose Frequently encountered, almost never fatal unless combined with other CNS depressants (if coma is present look for other drugs as well, especially ethanol) Antidote is flumazenil, which is a competitive antagonist, rarely used due to lack of overt toxicity Flumazenil is contraindicated when patients co-ingest tricyclic antidepressants due to potential for precipation of seizures 9. OPIATES Triad of coma, decreased respiration and pinpoint pupils suggests opiate overdose Naloxone is competitive antagonist, administer 0.4 to 2 mg iv every 2-3 min until improvement (up to 10 mg); if no improvement is observed consider alternate diagnosis; important to remember that naloxone has a shorter half-life than other opiates; patients can improve dramatically and want to be discharged, but should be evaluated after 24-48 hours This class of drugs includes: morphine, codeine, hydrocodone, hydromorphone, as well as others 224 10. COCAINE Routes of administration: insufflated (snorted), smoked (free base), intravenous and oral (buccal mucosal) Mechanism of action is blockade of norepinephrine reuptake and blockade of fast sodium channels, a local anesthetic effect Toxicity is due to excessive norepinephrine, beta-1 receptor activation (increase in heart rate, contractility and conduction velocity) and alpha-1 receptor activation (vasoconstriction, increase in blood pressure and ischemia), leading to: unidirectional heart block and reentry, coronary artery disease and myocardial infarctions Can become tolerant to stimulant effects, but not local anesthetic effects which can cause complete heart block Usually detected as inactive metabolite benzoylecgonine in urine specimens Active metabolite ethylbenzoylecgonine (cocaethylene) formed by transesterification with ethanol, if present; longer serum half-life than parent drug Treatment for cocaine overdose Stabilize the patient; if the patient reaches the hospital alive, prognosis is good due to rapid metabolism IV diazepam for agitation and seizures Ice baths for hyperthermia 11. AMPHETAMINES Methamphetamine (crystal) very prevalent in San Diego (“Meth Capital of the World”) Routes of administration include: smoking (free base form), intravenous and oral Mechanism of action is to release catecholamines from synaptic vesicles, block reuptake and some direct effects on CNS receptors Toxicity includes: hypertension, arrhythmias, agitation, hyperthermia, paranoia Street grade often contaminated with adulterants (ephedrine, phenylpropanolamine, etc.) Death usually not immediate but several hours after administration Treatment is supportive 12. BARBITURATES Sedative/hypnotic Different kinetics for individual barbiturates are important; short-acting (pentobarbital, secobarbital) are more potent than long-acting (phenobarbital) Necessary to identify type to interpret blood concentrations Not seen very often anymore (used to be a major concern), as benzodiazepines have replaced them as the first line drug Death due to respiratory arrest 225 13. PHENCYCLIDINE (PCP) Localized to certain region of the country (Washington DC) A dissociative veterinary anesthetic Patients can be very combative (treat with major tranquilizer such as haloperidol) Positive drug screen results should be confirmed by gas chromatography/mass spectrometry due to the low prevalence of this drug 14. HALLUCINOGENS (LSD, MESCALINE, MARIJUANA, ETC.) Not detected by most routine toxicology screens Generally not frankly toxic, behavioral toxicity more common (e.g., dangerous behavior) Analysis of powders, drug-impregnated papers, pills sometimes useful in helping determining type of exposure 15. DESIGNER DRUGS (FENTANYL ANALOGS, MDA, MDMA, ETC.) Originally synthesized to bypass DEA regulations Fentanyl analogs caused several deaths due to potency (e.g., “China White,” alphamethylfentanyl); can be treated with naloxone MDA and MDMA (“Ecstasy,” XTC) are amphetamine analogs with similar toxicity, yet cause a long term toxicity to serotonin containing neurons Designer meperidine drug caused severe Parkinson’s disease (MPTP) 16. DIGITALIS (DIGOXIN) Cardiac drug with narrow therapeutic index, used to treat congestive heart failure (not a first line drug) Inhibits sodium/potassium ATPase and increases force of contraction Toxicity is characterized by A/V block, nausea/vomiting Overdose treated with digoxin FAB antibody fragments (Digibind) which inactivate digoxin FAB fragments can crossreact with immunoassays designed to detect digoxin, so interpretation of laboratory values when giving Digibind can be confusing Monitor digoxin and serum K+ concentration (K+ increases with toxicity) in an overdose situation 17. CARBON MONOXIDE (FOR CONCENTRATION EFFECT SEE TABLE 1) The most common cause of fatal chemical poisoning (primarily due to smoke inhalation) Colorless, odorless, tasteless gas Has 240 times the affinity for hemoglobin than oxygen (yields carboxyhemoglobin, COHb) Normal nonsmokers 1-2% COHb, smokers 5-6% COHb Treatment is fresh air, 100% O2 and possibly hyperbaric oxygen 226 Table 1: Blood Carbon Monoxide - Concentrations and Effects % COHb 10 20 30 40-50 50 -70 70-80 Effect* Shortness of breath with vigorous exercise, dilation of cutaneous vessels Shortness of breath, headache Irritability, dizziness, nausea Confusion, collapse Syncope, coma Death * Greatly dependent on degree of activity 18. IRON Most cases are due to children ingesting vitamin preparations with iron Causes metabolic acidosis by a complex mechanism involving uncoupling of oxidative phosphoralation, renal failure, and formation of unbuffered hydrogen ions when iron is hydrated A) Iron toxicity often has 4 phases Stage I (immediate effects): may include: vomiting, diarrhea, abdominal pain, metabolic acidosis, hyperglycemia Stage II (6 and 24 hours post ingestion): hypovolemia, hypotension, metabolic acidosis, serum concentrations may not have peaked yet Stage III (12 to 24 hours post ingestion): multiple organ failure (GI, CNS, hepatorenal, coagulopathies, hypoglycemia); fulminant hepatic failure is commonly fatal Stage IV (4-6 weeks post ingestion): gastric scarring and pyloric obstruction Overdoses cause gastrointestinal corrosion B) Treatment for iron poisoning Serial monitoring of serum iron; maximum concentrations can occur up to 24 hours post-ingestion Obtain creatinine, electrolytes, hemoglobin, prothrombin time, liver function tests and arterial blood gases; a positive radiograph confirms diagnosis, but a negative radiograph does not exclude overdose Calculate elemental iron dose ingested; between 20 and 60 mg Fe/kg poses moderate risk, > 60 mg/kg has high risk for toxicity < 350 g/dl and no symptoms, no deferoxamine; use supportive care > 300 g/dl and symptomatic, give deferoxamine (15 mg/kg/hr) by continuous intravenous infusion Deferoxamine chelates iron into a form that is rapidly excreted unchanged in urine (half-life of 6 hours), imparting a “vin rose” color to the urine 227 19. LEAD (FOR CONCENTRATION EFFECT LEVEL SEE FIGURE 9) Essentials (from Ford, M.D. et al., Clinical Toxicology, 1st edition, Philadelphia: Saunders, 2001) Multisystemic signs and symptoms include headache (in severe cases encephalopathy), abdominal pain, anemia, and, less commonly, gout, motor neuropathy, and renal insufficiency Subclinical effects in children include neurocognitive deficits, growth retardation, and developmental delay Laboratory tests may show anemia and basophilic stippling; definitive diagnosis is made by elevated blood lead concentration Lead summary One of the most common devastating environmental diseases of young children Demyelinates nerve fibers causing peripheral neuropathy (wrist drop) and encephalopathy Causes decreased IQ and developmental disturbances in children Inhibits Fe incorporation into heme, and thus increases free erythrocyte protoporphyrin (FEP) which has been used to measure exposure; however, FEP generally is not as sensitive as blood lead levels Complex cellular toxicity of lead due to interaction with calcium in cellular signaling leading to neuropathies as well as hypertension Chronic lead poisoning causes hypochromic anemia, with basophilic stippling Whole blood lead concentrations are used to triage patients, as 99% of circulating lead is in the red cells 90% of adult body burden of lead is stored in bones Most common form of treatment is EDTA (versenate), which chelates lead; the chelation product is excreted into the urine Treatment for lead poisoning Optimal treatment of lead intoxication combines decontamination, supportive care, and judicious use of chelating agents CDC recommends children with blood lead >45 ug/dL get chelation therapy; Adults with elevated blood lead and symptoms of lead toxicity should receive chelation therapy Chelation therapy includes CaNa2-EDTA intravenous Some clinicians advise including dimercaprol (BAL) 3-5 mg/kg IM with EDTA therapy Monitor blood lead 24-48 hours after chelation to monitor for rebound lead concentrations Multiple rounds of chelation therapy are often necessary 228 20. ORGANOPHOSPHATE OR CARBAMATE OVERDOSE Used as insecticides Patients present with SLUD (salivation, lacrimation, urination, and defecation) due to excessive cholinergic stimulation Treat with atropine until drying of pulmonary secretions Pralidoxime can be used with atropine for severe organophosphate poisonings (functions by reactivating cholinesterases; best if given within 24 hours) Lowest observed effect Levels of Pb in children Pb (g/dL) 150 Death 100 Encephalopathy, coma, convulsions Nephropathy Anemia 50 40 Hemoglobin synthesis 20 Nerve conduction velocity Erythrocyte protoporphyrin 10 Developmental toxicity ( IQ, hearing, growth) Figure 9 229 F. CASE STUDIES CASE 1 A 23-year-old male patient presented to the emergency department smelling of alcohol and in an incoherent state with significant ataxia. His initial laboratory findings included: Lab Arterial blood gas (pH) pCO2 (mmHg) Lactic acid (mmol/L) Plasma ethanol (mg/dL) Glucose (mg/dL) Sodium (mmol/L) SUN (mg/dL) Osmolality (mOsm/kg) Calcium (mg/dL) Potassium (mmol/L) Chloride (mmol/L) HCO3 (mmol/L) Result Reference Range 6.85 (7.35-7.45) 14 (35-45) 30.3 (0.7-2.1) 217 Negative 63 (70-110) 129 (135-145) 30 (8-23) 376 (270-310) 8.2 (8.4-10.2) 4.3 (3.4-4.8) 78 (95-107) 5 (22-28) 1. Calculate this patient’s osmol gap. 2. How much osmolality does the ethanol account for? 3. What are common causes of elevated osmolality? 4. Calculate this patient’s anion gap. 5. What potential toxin could explain these results? 6. What additional laboratory tests would you like to order? 7. What therapeutic options would you suggest? 230 CASE 2 A 32-year-old male with history of depression, alcoholism, hepatitis C, and a past suicide attempt presented at 20:30 to the emergency department with acute Tylenol/Benadryl overdose. The patient reported taking 100 pills of Tylenol PM (acetaminophen 500 mg and Benadryl 25mg) at about 20:00. The admission laboratory findings included: WBC 19.2 x 103 cells/mL with 60% segs, few bands, Hgb 14.1, Hct 41.4 g/dL Na 141 mEq/L, K 3.9 mEq/L, Cl 106 mEq/L, total CO2 25 mmol/L, SUN 4 mg/dL, Creatinine 0.7 mg/dL, Glucose 95 mg/dL Ca 8.7 mg/dL, Liver function tests were within normal limits as were coagulation studies The admission serum acetaminophen concentration was 40 ug/mL. 1. How should this patient be treated? 2. Does this patient’s past medical history have any impact on his therapy? After being in the emergency department for 3 hours his acetaminophen concentration rises to 175 ug/mL. 3. How do you interpret this concentration using the Rumack diagram and what does this tell you about potential toxicity? After being in the emergency department for 5 hours his acetaminophen concentration rises to 192 ug/mL, and after 10 hours the acetaminophen concentration is 105 ug/mL. 4. What do the excretion kinetics demonstrate with regards to potential acetaminophen toxicity? 5. What other laboratory tests would be monitored in this case? 231 CASE 3 (South. Med. J. 98(2):241-244, 2005) An 18-year-old woman who weighed 61 kg was admitted to a community hospital, 4 to 5 hours after a one-time ingestion of approximately 100 ferrous sulfate pills (200 mg of FeS0 4 per pill). The patient had had an altercation with a friend, and the overdose was with suicidal intent. Shortly thereafter, she had nausea and one episode of scant hematemesis with epigastric pain and one episode of watery diarrhea. The emergency medical service personnel brought her to the hospital after being called by the patient. At admission, the patient vomited visible pill fragments. Laboratory Parameters Serum creatinine (normal range: 0.5-1.5 mg/dL) Serum bicarbonate (normal range: 23-27 mmol/L) Serum albumin (normal range: 3.5-4.5 g/dL) Total bilirubin (normal range: 0.0-1.0 mg/dL) Conjugated bilirubin (normal range: 0.0-0.3 mg/dL) Alkaline phosphatase (normal range: 35-129 U/L) Serum alanine aminotransferase (normal range: 4-41 U/L) Serum AST (normal range: 4-37 U/L) PTT (normal range: 24.7-35.8 seconds) Prothrombin time (normal range: 12.8-14.8 seconds) International normalized ratio Platelets (normal range: 150-400 k/mm3) White blood cell count (normal range: 3.5-10.5 k/mm3) Iron (normal range: 37-158 ug/dL) Day 1 0.7 20 3.9 0.3 0.1 59 Day 2 Day 3 Day 4 0.7 0.6 0.4 19 19 22 3.0 3.0 2.7 3.1 2.3 2.3 1.6 1.2 1.5 87 84 85 14 17 34.4 14.5 1.0 225 4,048 3,618 2,642 2,417 1,993 630 35.3 39.5 33.8 46.3 51.5 38.8 5.0 5.8 4.0 166 165 164 8.4 340 1. How much elemental iron did this patient ingest? 2. Is this a potentially toxic dose of iron? 3. How should this patient be treated? 4. How do you interpret her laboratory values on day 2? 5. How would you expect her serum to appear on day 2? 6. What other laboratory studies would be indicated? 7. Is this a typical presentation for an iron overdose? 232 7.8 136 6.9 219 9.3 207 XVIII THERAPEUTIC DRUG MONITORING 233 234 A. OBJECTIVES To understand peak and trough monitoring aminoglycoside antibiotics To understand why TDM is indicated for various drugs To be able to recognize common symptoms of toxicity for therapeutic drugs To introduce the use of pharmacogenetics in therapeutic drug monitoring B. KEY TERMS Therapeutic drug monitoring - a strategy where a patient’s dosing schedule is modified based on measurement of serum drug concentrations Pharmacogenetics - the study of the hereditary basis for differences in a population’s drug response Half-life - the time it takes for the plasma concentration of a drug to decrease by fifty percent Pharmacokinetics - a description of the time course of a drug in the body, includes processes of absorption, distribution, and elimination Pharmacodynamics - the processes of interaction of pharmacologically active substances with target sites, and the biochemical and physiological consequences leading to therapeutic or adverse effects Mechanism of action - the biochemical or physical process occurring at the site of action to produce a pharmacological effect C. BACKGROUND/SIGNIFICANCE The value of therapeutic drug monitoring (TDM) as an adjunct to rational drug therapy in patients with various diseases has been firmly established. The clinician who monitors a patient’s serum drug concentration is in a position to know why a patient either is not responding satisfactorily to a particular drug dosage or is experiencing side effects to a standard therapeutic dosage of a drug. Without question, TDM has significantly improved patient care. For example, more than 80% of all epileptic inpatients can have their disorder controlled with a single drug at appropriate dosages if the concentrations of that drug in plasma are routinely monitored. This contrasts sharply with previous dosage regimens for the epilepsies, which almost invariably involved administration of at least two or three drugs to produce the desired anticonvulsant effect. TDM ADVANTAGES: Noncompliance can be identified Individual variations in drug-disposition patterns can be dealt with appropriately Altered drug utilization as a consequence of disease can be readily identified Compensation for an altered physiological state (e.g., age related changes in metabolism) Drug interactions, can be identified 235 BEFORE ORDERING TDM, ANSWER THE FOLLOWING QUESTIONS: 1) What therapeutic information can be derived from this test (e.g., compliance, altered pharmacokinetics, toxicity)? 2) What are the concentrations for therapeutic and toxic effects? 3) What are the clinical effects with serum concentrations higher or lower than the therapeutic range? 4) Does the drug have a narrow therapeutic range? 5) What is the specificity of the assay? 6) What is the time relationship between dose and sample collection? 7) Does the clinical value of these results justify their cost? D. THERAPEUTIC MONITORING OF SPECIFIC DRUGS 1. PHENYTOIN TDM necessary because drug often exhibits saturated kinetics at therapeutic doses Antiepileptic, modulates synaptic sodium channels by prolonging inactivation Anti-epileptic therapeutic concentrations range from 10 to 20 mg/L 90-95% protein bound Apparent half-life 8 to 60 hours (dose dependant) Difficult to determine when steady state is reached (5 to 30 days) Time for collection is dictated by reason for monitoring, monitor peak for toxicity (48 hours after oral dose) and trough (just before next dose) to ensure adequate therapeutic response Dose adjustment is primarily empirical Toxicity characterized by: nystagmus, drowsiness, ataxia, confusion, tremors, and seizures Treat toxicity by limiting drug A common adverse effect with chronic use is gingival hyperplasia (40-50% prevalence) 2. THEOPHYLLINE A methylxanthine used to treat difficult cases of asthma (a third-line therapy) Inhibits cyclic nucleotide phosphodiesterase enzymes (PDE’s), leading to an increase in cAMP and cGMP and is a competitive antagonist at adenosine receptors, causing relaxation of bronchial smooth muscles Causes an increase in circulating concentrations of catecholamines Pharmacokinetics are age dependent (average half-life about 3.5 hours in children, 89 hours in adults) Phenytoin and phenobarbital increase theophylline clearance two-fold Primarily cleared by liver metabolism Therapeutic concentrations are in the range of 10 to 20 mg/L Overdose treated with activated charcoal and hemodialysis or charcoal hemoperfusion Chronic intoxication more severe than acute Toxicity characterized by nausea, vomiting, hypokalemia, hyperglycemia, seizures, tachycardia, anion gap, metabolic acidosis 236 3. VALPROIC ACID (DEPAKENE OR DEPAKOTE) Inhibits GABA transaminase, therefore increasing concentrations of GABA Rapidly and completely absorbed after oral administration Peak concentrations occur 1-4 hours post dose Half-life varies with age (16 hours in healthy adults, 8 hours in children), duration of therapy (adult chronic therapy, half-life of 12 hours), and hepatic disease Therapeutic range 50-100 mg/L (trough) Concentrations above 100 mg/L are associated with hepatic toxicity and acute toxic encephalopathy 4. PHENOBARBITAL Hyperpolarizes GABAa receptor Slowly but completely absorbed (4 to hours peak concentration after oral dose) Long elimination half-life (70 to 100 hours) Therapeutic range 10-40 mg/L (trough) Toxicity includes: sedation, ataxia, slurred speech, and CNS depression 5. PRIMIDONE (MYSOLINE) Active metabolite phenobarbital contributes to pharmacological effect, need to measure parent drug and phenobarbital Rapidly and completed absorbed after oral dose Half-life of about 10 hours Therapeutic range 5-12 mg/L (trough) Toxicity includes: nausea, vomiting, dizziness, ataxia, and CNS depression 6. CARBAMAZEPINE (TEGRETOL) Slowly and erratically absorbed Highly protein bound (80%) Elimination half-life changes with duration of therapy (initially 24 hours, reduces to 15- 20 hours with therapy) Therapeutic range 8-12 mg/L (trough) Active metabolite carbamazepine-10-11-epoxide accumulates in children Toxicity characterized by blurred vision, nystagmus, and ataxia Toxicity unrelated to dose includes hematological depression (leukopenia, thrombocytopenia and aplastic anemia) 7. ETHOSUXIMIDE (ZARONTIN) Readily absorbed from gastrointestinal tract Half-life of 33 hours Therapeutic range 40-100 mg/L Toxicity characterized by gastrointestinal distress, lethargy, and dizziness 237 8. DIGOXIN (LANOXIN) Cardiac glycoside that inhibits Na/K-ATPase Variably absorbed, 25% protein bound Therapeutic range 0.8-2.0 ng/mL Critical that serum is drawn as a trough (at minimum 8 hours post dosing) Toxicity characterized by: nausea, vomiting, green/yellow visual disturbance, premature ventricular contractions, ventricular tachycardia, and atrioventricular node block Analytically difficult to measure due to multiple metabolites and cross-reactivity problems 9. PROCAINAMIDE (PRONESTYL) Antiarrhythmic with active metabolite N-acetylprocainamide (NAPA) Therapeutic concentration of procainamide plus NAPA 5-30 mg/L Fast acetylators form more NAPA which accumulates in plasma NAPA also accumulates in renal failure Symptoms of intoxication include: bradycardia, prolongation of the QRS interval, AV block and arrhythmias 10. AMINOGLYCOSIDES Includes: amikacin, gentamicin, tobramycin Poorly absorbed orally, consequently routinely administered intravenously or intramuscularly Therapeutic concentrations listed in Table 1 Draw peak to determine therapeutic response, trough for toxicity Toxicity: ototoxic and nephrotoxic Primarily renally cleared 11. CYCLOSPORINE Effective in suppressing host vs. graft rejection in organ transplants Highly variable absorption Whole blood concentration correlates well with immunosuppression and toxicity Therapeutic range for renal transplant 100-300 ng/mL Therapeutic range for cardiac, hepatic and pancreatic transplants 200-350 ng/mL Levels exceeding therapeutic concentrations associated with renal toxicity 12. VANCOMYCIN Complex lycopeptide antibiotic effective against gram positive organisms Ototoxic and nephrotoxic 90% of intravenous dose is excreted renally Target trough concentration are 5-15 mg/L 238 13. SIROLIMUS Indicated for the prophylaxis of organ rejection in patients receiving renal transplants Inhibits T-lymphocyte activation and proliferation in response to antigenic and cytokine stimulation Sirolimus also inhibits antibody production Recommended that sirolimus initially be used in combination with cyclosporine and corticosteroids In patients with low to moderate immunological risk, cyclosporine should be withdrawn 2-4 months after transplantation and sirolimus dose should be increased to reach recommended blood concentrations Target trough concentrations (whole blood) 4-12 ng/mL (in combination with cyclosporine) for kidney transplants Excreted primarily in feces, elimination half-life of 62 hours Adverse effects include: hypercholesterolemia, hypertriglyceridemia, renal dysfunction, pneumocystis carinii infection, and CMV infection 14. TACROLIMUS Indicated for the prophylaxis of organ rejection in allogeneic liver or kidney transplants Recommended for use concomitantly with adrenal corticosteroids Inhibits T-lymphocyte activation Target trough concentration are 5-20 ng/mL (whole blood) Primary route of excretion is fecal (> 90% of dose) Elimination half-life of 48 hours Nephrotoxic (approximately 50% of kidney transplantation patients and 40% of liver transplants) Should not be used simultaneously with cyclosporine Causes mild to severe hyperkalemia (10-40% of patients), therefore monitor serum potassium) Causes neurotoxicity, including; tremor, headache, changes in motor function, mental status, etc. Other toxicities include: hypertension, increased risk of cancer, insulin dependant diabetes mellitus E. PHARMACOGENETICS AND TDM Genetic differences in cytochrome P450 (CYP) enzymes responsible for altered bioavailability of drugs Three main CYP families responsible for most drug metabolism are CYP1, CYP2 and CYP3 Future directions of TDM will undoubtedly incorporate more genetic analysis of both CYP enzymes and drug transporter proteins Pharmacogenomics, where a patient’s single nuclear polymorphisms (SNP’s) are tested to determine which drugs will provide the optimal response will also enhance clinical therapeutics in next several years. 239 Principle of patient “titration” - a caution against rigid adherence to the “therapeutic range.” F. TABLE 1: DATA FOR SOME COMMONLY MONITORED THERAPEUTIC DRUGS t1/2 Dose Interval Therapeutic See Drug (hours) (hours) Range Critical notes 1-3 4 10-25 mg/L > 40 mg/L Acetaminophen peak 20-30 mg/L 2.5 6-8 > 40 mg/L 1 Amikacin trough 1-8 mg/L 10-48 8 8-12 mg/L > 15 mg/L Carbamazepine trough 0.8-2.0 33-51 24 > 2.5 ng/mL 2 Digoxin ng/mL peak 4-10 mg/L 2 8 > 12 mg/L 1 Gentamicin trough 1-2 mg/L 1.8 Infusion 1.5-5 mg/L > 8.0 mg/L Lidocaine 19 6-12 0.5-1.5 mmol/L > 2.0 mmol/L Lithium PA + NAPA 6-12 n/a 5-30 mg/L 3 NAPA > 35 mg/L 72-100 12 10-40 mg/L > 60 mg/L Phenobarbital 6-24 8 10-20 mg/L > 25 mg/L Phenytoin 6-12 6-8 5-12 mg/L > 15 mg/L 4 Primidone PA 4-10 mg/L NAPA 5-30 mg/L PA + NAPA Procainamide 3-5 4-6 3 PA + NAPA 5-30 > 35 mg/L (PA) mg/L trough 2.3-5.0 4-7 6 > 7 mg/L Quinidine mg/L 2-19 4 10-30 mg/dL > 45 mg/dL Salicylate 3-9 Theophylline 10-20 g/mL > 30 g/mL peak 4-10 mg/L > 12 mg/L 2 8 trough 0.5-1.5 1 Tobramycin >5 mg/L mg/L 8-15 8 50-100 mg/L > 200 mg/L Valproic acid peak 30-40 mg/L > 50 mg/L 5.0-6.5 6-12 1 Vancomycin trough 5-15 mg/L > 15 mg/L NOTES: 1) Draw trough immediately prior to next dose - For intramuscular dose, draw peak 45-60 minutes post dose - For 30 minute intravenous infusion, draw peak 30 minutes post dose - For 60 minutes intravenous infusion, draw peak 15 minutes post dose 2) Draw specimen > 8 hours after dosing 3) N-acetylprocainamide (NAPA) is the active metabolite of procainamide (PA); it has similar pharmacological effects as the parent compound; both should be monitored 4) Phenobarbital is the active metabolite of primidone and the dose is usually titrated to obtain therapeutic concentrations of phenobarbital 240 G. CASE STUDIES CASE 1 A 71-year-old male with a history of orthotopic (transplanted in normal anatomic position) liver transplant 8 years prior had been taking cyclosporine chronically. The patient presents to the emergency department following a syncopal episode. His admission laboratory findings include: Na 137, Cl 109, CO2 19, SUN 40, creatinine 2.3, glucose 159, Ca 9.2, albumin 3.0, ALK phos 202, total protein 6.5 g/dL, total bilirubin 1.2 mg/dL, direct bilirubin 0.3 mg/dL, AST 33 U/L, ALT 69 U/L. 1. How do you interpret these baseline data? While in the hospital the patient was given two doses of ibuprofen for pain. Over the next several days his SUN and creatinine increased to 80 mg/dL and 4.3 mg/dL respectively. The patient became confused and displayed obvious tremors. 2. How do you explain the increases in SUN and creatinine? 3. What is the appropriate therapy for this patient? 4. What would you expect to happen to the concentration of cyclosporine in this setting? 5. Why was this patient becoming confused? 6. Is this a typical drug interaction between these two drugs? 241 CASE 2 A 46-year-old male with a history of chronic back pain of 7 years duration presents to the pain clinic seeking relief. His pain is 8-9 out of 10 and is somewhat relieved with vicodin (1 tablet as needed) and salsalate 750 mg TID. He suffers from constipation and complains that his pain is “burning and shooting” in nature. His internal medicine physician prescribes him 200 mg carbamazepine BID to help relieve pain. Six weeks later his carbamazepine is increased to 600 mg BID. Two weeks after his carbamazepine is increased to 600 mg BID the patient is brought to the emergency department by a friend who reported the patient was falling down and passing out while at work. The patient also complained of excessive sweating and nausea. His physical exam showed lateral nystagmus in both eyes and slight orthostasis, but was otherwise unremarkable. In the emergency department a stat serum carbamazepine concentration was 14.4 mg/L. 1. Was this patient’s carbamazepine dosage monitored correctly? 2. Could the patient’s symptoms (dizziness, nausea, etc.) be explained by his medications? 3. Was this a preventable adverse reaction? 4. Is this a common use for carbamazepine? 5. How should this patient be treated? 6. In addition to monitoring his carbamazepine concentration what other laboratory tests should be monitored? 242 CASE 3 (Clin. Pharmacol. Ther. 70(4):391-394, 2001) A 31-year-old female (weight 55 kg; height, 159 cm) was admitted following a car accident which had caused significant head trauma. Two months after the accident and ten days after starting a standard dose of oral phenytoin (100 mg tid) the patient manifested dysarthria, nystagmus, dysmetria, left hemifacial dyskinesia and alterations in mental status 3 hours after taking her phenytoin. The only other medication the patient was taking at this time was a low molecular weight heparin (nadroparin, 4000 IU per day, subcutaneously). A cerebral computed tomographic scan and electroencephalogram excluded possible late consequences of the head trauma. A stat phenytoin concentration was > 100 mg/L. 1. How do you interpret this phenytoin concentration? 2. How would you treat this patient? 3. How would you explain this phenytoin concentration? 4. What additional studies would you order to test your hypothesis? 243 CASE 4 A 51-year-old male with seizure disorder presents with change in mental status x4 days. The patient is confused and disoriented with an unreliable history. His history is provided by his wife. Confusion and disorientation worsened 4 days ago, associated with slurred speech. The patient also experienced several falls in last couple of days along with seizures and complaints of right hip pain. His wife reports withholding his medication for the last 2 days after noticing signs and symptoms of toxicity. The patient has difficulty taking medications and has had multiple admissions for supratherapeutic and subtherapeutic phenytoin levels. Active problem(s): Hypothyroidism Alcohol abuse-continuous Seizure disorder Diabetes with neurological manifestations, type II Labs: Serum urea nitrogen (mg/dL) Albumin (g/dL) Glucose (mg/dL) Creatinine (mg/dL) EGFR (ml/min) Sodium (mMol/L) Potassium (mMol/L) Chloride (mMol/L) CO2 (mMol/L) Calcium (mg/dL) PO4 (mg/dL) Free T-4 (ng/dL) TSH (uIU/mL) Phenytoin (mg/dL) Result Ref. Range 14 8-23 3.6 3.2-4.6 101 70-110 0.8 0.4-1.2 108 94-140 139 135-145 4.0 3.5-5.0 104 95-106 28.0 24-31 8.7 8.4-10.2 3.8 2.5-4.5 0.6 0.7-1.9 4.87 0.49-4.67 47.3 10-20 Complete blood count showed mild macrocytic anemia. 1. What additional labs would you order on this patient? 2. Are these signs and symptoms consistent with the thyroid function studies? 3. Are these signs and symptoms consistent with phenytoin toxicity? 4. How should this patient be treated? 244 5. How long will it take for his phenytoin to return to therapeutic concentration? 6. What is the most likely cause of the macrocytic anemia? 245 CASE 5 A 75-year-old obese man with a history of diabetes mellitus (type II), asthma, gout, and atrial fibrillation presented to urgent care with worsening dyspnea on exertion. On initial presentation the patient was alert, oriented, and in no distress. His theophylline dose had recently been increased from 600 to 800 mg per day. EKG at the time revealed atrial fibrillation at 176 BPM. The patient claims to have been using albuterol 4-6 puffs q2 hours and theophylline (6 tabs of 200 mg each in last four hours, 5-6 tabs/day x3d). The patient suffered a grand mal seizure in urgent care and a code blue was called. Continued seizure activity was noted even after the patient was given phenytoin and lorazepam. The patient given pentobarbital and occasional twitches were noted. Test name Blood urea nitrogen (mg/dL) Albumin (g/dL) Glucose (mg/dL) Creatinine (mg/dL) EGFR (mL/min) Sodium (mMol/L) Potassium (mMol/L) Chloride (mMol/L) CO2 (mMol/L) Calcium (mg/dL) PO4 (mg/dL) BNP (pg/dL) Theophylline (mg/L) Result Ref. Range 31 8-23 3.6 3.2-4.6 188 70-110 1.4 0.4-1.2 53 94-140 134 135-145 3.2 3.5-5.0 95 95-106 20.0 24-31 9.5 8.4-10.2 3.3 2.5-4.5 569 0-100 56 10-20 1. How do you interpret this laboratory data? 2. What additional laboratory studies are indicated? 3. How would you treat this patient? 4. Could this overdose have been prevented? 246 GLOSSARY 247 248 A achlorhydria: absence of hydrochloric acid from the gastric juice acidosis: a pathologic state characterized by an increase in the concentration of hydrogen ions in the arterial blood above normal level, 40 nmol/L, or pH 7.4 acute coronary syndrome (ACS): a wide range of acute heart conditions including STsegment elevation myocardial infarction, non-ST-segment myocardial infarction and unstable angina acute myocardial infarction (AMI), also myocardial infarction (MI): infarction of a segment of the heart muscle, usually as a result of occlusion of a coronary artery acute pancreatitis: an acute inflammation of the pancreas accompanied by the formation of necrotic areas and hemorrhage into the substance of the gland; clinically marked by sudden severe abdominal pain, nausea, fever, and leukocytosis; areas of fat necrosis are present on the surface of the pancreas and in the omentum because of the action of the escaped pancreatic enzyme (trypsin and lipase) adenoma: a benign epithelial neoplasm in which the tumor cells form glands or gland-like structures; usually well circumscribed, tending to compress rather than infiltrate or invade adjacent tissue adrenocorticotrophic hormone (ACTH): a 39 amino-acid peptide hormone secreted by anterior pituitary that acts primarily on the adrenal cortex stimulating its growth and its secretion of corticosteroids alanine aminotransferase (ALT): an enzyme transferring amino groups from l-alanine to 2ketoglutarate, or the reverse (from l-glutamate to pyruvate); there is a d-alanine transaminase that effects the same reaction, but using d-alanine and d-glutamate; serum concentration is increased in viral hepatitis and myocardial infarction alkaline phosphatase: a phosphatase with an optimum pH of above 7.0 (e.g., 8.6), present ubiquitously; localized cytochemically in membranes by modifications of Gomori nonspecific alkaline phosphatase stain; it hydrolyzes many orthophosphoric monoesters; low levels of this enzyme are seen in cases of hypophosphatasia alkalosis: a state characterized by a decrease in the hydrogen ion concentration of arterial blood below normal level, 40 nmol/L, or Ph 7.4; the condition may be caused by an increase in the concentration of alkaline compounds, or by a decrease in the concentration of acidic compounds or carbon dioxide amylase: one of a group of amylolytic enzymes that cleave starch, glycogen, and related 1,4α-glucans anemia: any condition in which the number of red blood cells per mm3, the amount of hemoglobin in 100 ml of blood, and/or the volume of packed red blood cells per 100 ml of 249 blood are less than normal; clinically, generally pertaining to the concentration of oxygentransporting material in a designated volume of blood; anemia is frequently manifested by pallor of the skin and mucous membranes, shortness of breath, palpitations of the heart, soft systolic murmurs, lethargy, and fatigability anion gap: the difference between the sum of the measured cations and anions in the plasma or serum calculated as follows: (Na+ + K+) - (Cl- + HCO-3); elevated values may occur in diabetic or lactic acidosis; normal or low values occur in bicarbonate-losing metabolic acidoses anuria: absence of urine formation aplastic anemia: anemia characterized by a greatly decreased formation of erythrocytes and hemoglobin, usually associated with pronounced granulocytopenia and thrombocytopenia, as a result of hypoplastic or aplastic bone marrow apolipoproteins: the protein component of any lipoprotein complexes apotransferrin: plasma iron binding protein with no iron bound aspartate aminotransferase (AST): an enzyme catalyzing the reversible transfer of an amine group from l-glutamic acid to oxaloacetic acid, forming α-ketoglutaric acid and laspartic acid; a diagnostic aid in viral hepatitis azotemia: an abnormal increase in concentration of urea and other nitrogenous substances in the blood plasma B B cell lymphocyte: an immunologically important lymphocyte that is not thymus-dependent, is of short life, and resembles the bursa-derived lymphocyte of birds in that it is responsible for the production of immunoglobulins, i.e. it is the precursor of the plasma cell and expresses immunoglobulins on its surface but does not release them; it does not play a direct role in cell-mediated immunity basophil: a cell with granules that stain specifically with basic dyes; a phagocytic leukocyte of the blood characterized by numerous basophilic granules containing heparin and histamine and leukotrienes; except for its segmented nucleus, it is morphologically and physiologically similar to the mast cell though they originate from different stem cells in the bone marrow Bence Jones protein: proteins with unusual thermosolubility found in the urine of patients with multiple myeloma, consisting of monoclonal immunoglobulin light chains benign: denoting the mild character of an illness or the nonmalignant character of a neoplasm 250 bilirubin: a yellow bile pigment found as sodium bilirubinate (soluble), or as an insoluble calcium salt in gallstones; formed from hemoglobin during normal and abnormal destruction of erythrocytes by the reticuloendothelial system; excess bilirubin is associated with jaundice C cancer: general term frequently used to indicate any of various types of malignant neoplasms, most of which invade surrounding tissues, may metastasize to several sites, and are likely to recur after attempted removal and to cause death of the patient unless adequately treated; especially, any such carcinoma or sarcoma, but, in ordinary usage, especially the former cardiac markers: proteins used to monitor damage to cardiac tissue, typically cTnI, cTnT, CKMB, and Myoglobin cholestasis: an arrest in the flow of bile; cholestasia due to obstruction of bile ducts is accompanied by formation of plugs of inspissated bile in the small ducts, canaliculi in the liver, and elevation of serum direct bilirubin and some enzymes chronic pancreatitis: repeated exacerbations of pancreatitis in patient with chronic inflammation of that organ; relapses are usually due to persistence of etiologic factor or repeated exposure to it, such as occurs with partial ductal obstruction or chronic alcoholism chylomicron: a large lipid droplet of reprocessed lipid synthesized in epithelial cells of the small intestine and containing triacylglycerols, cholesterol esters, and several apolipoproteins cirrhosis: end stage liver disease characterized by diffuse damage to hepatic parenchymal cells, with nodular regeneration, fibrosis, and disturbance of normal architecture; associated with failure in the function of hepatic cells and interference with blood flow in the liver, frequently resulting in jaundice, portal hypertension, ascites, and ultimately biochemical and functional signs of hepatic failure coagulation: clotting; the process of changing from a liquid to a solid, said especially of blood (i.e., blood coagulation); in vertebrates, blood coagulation is a result of cascade regulation from fibrin coefficient of variation: the ratio of standard deviation to the mean congenital: existing at birth, referring to certain mental or physical traits, anomalies, malformations, diseases etc. which may be either hereditary or due to an influence occurring during gestation up to the moment of birth congenital adrenal hyperplasia: a group of autosomal recessively inherited disorders associated with a deficiency of one of the enzymes involved in cortisol biosynthesis, resulting in elevation of ACTH levels and overproduction and accumulation of cortisol precursors proximal to the block; androgens are produced in excess, causing virilization; the most common disorder is the 21-hydroxylase deficiency, caused by mutation in the 251 cytochrome P450 21-hydroxylase gene (CYP21) on chromosome 6p; there are four major types with some clinical similarities but distinctive genetic and biochemical differences: congestive heart failure: inadequacy of the heart so that as a pump it fails to maintain the circulation of blood, with the result that congestion and edema develop in the tissues corticosteroid: a steroid produced by the adrenal cortex (i.e., adrenal corticoid); a corticoid containing a steroid corticotrophin releasing hormone (CRH): a factor secreted by the hypothalamus that stimulates the pituitary to release adrenocorticotropic hormone creatinine: a component of urine and the final product of creatine catabolism; formed by the nonenzymatic dephosphorylative cyclization of phosphocreatine to form the internal anhydride of creatine creatine kinase (CK): an enzyme catalyzing the reversible transfer of phosphate from phosphocreatine to ADP, forming creatine and ATP; of importance in muscle contraction creatine kinase MB (CKMB): an enzyme catalyzing the reversible transfer of phosphate from phosphocreatine to ADP, forming creatine and ATP; of importance in muscle concentration; certain isozymes are elevated in plasma following myocardial infarctions cystic fibrosis: a congenital metabolic disorder in which secretions of exocrine glands are abnormal; excessively viscid mucus causes obstruction of passageways (including pancreatic and bile ducts, intestines, and bronchi), and the sodium and chloride content of sweat are increased throughout the patient’s life; symptoms usually appear in childhood and include meconium ileus, poor growth despite good appetite, malabsorption and foul bulky stools, chronic bronchitis with cough, recurrent pneumonia, bronchiectasis, emphysema, clubbing of the fingers, and salt depletion in hot weather; detailed genetic mapping and molecular biology have been accomplished by the methods of reverse genetics; autosomal recessive inheritance, caused by mutation in the cystic fibrosis conductance regulator gene (CFTR) on chromosome 7q D diabetes mellitus: chronic metabolic disorder in which utilization of carbohydrate is impaired and that of lipid and protein enhanced; it is caused by an absolute or relative deficiency of insulin and is characterized, in more severe cases, by chronic hyperglycemia, glycosuria, water and electrolyte loss, ketoacidosis, and coma; long-term complications include neuropathy, retinopathy, nephropathy, generalized degenerative changes in large and small blood vessels, and increased susceptibility to infection direct bilirubin: the fraction of serum bilirubin which has been conjugated with glucuronic acid in the liver cell to form bilirubin diglucuronide; so called because it reacts directly with the Ehrlich diazo reagent; increased levels are found in hepatobiliary diseases, especially of the obstructive variety 252 disseminated intravascular coagulation (DIC): a hemorrhagic syndrome that occurs following the uncontrolled activation of clotting factors and fibrinolytic enzymes throughout small blood vessels; fibrin is deposited, platelets and clotting factors are consumed, and fibrin degradation products inhibit fibrin polymerization, resulting in tissue necrosis and bleeding dyslipidemia: disorder of lipoprotein metabolism dyspnea: Shortness of breath, a subjective difficulty or distress in breathing E emergency toxicology: the laboratory diagnosis of the presence and severity of drug overdose, often in the comatose or obtunded patient eosinophil: a polymorphonuclear leukocyte characterized by many large or prominent, refractile, cytoplasmic granules that are fairly uniform in size and bright yellow-red or orange when treated with Wright or similar stains; the nuclei are usually larger than those of neutrophils, do not stain as deeply, and characteristically have two lobes (a third lobe is sometimes interposed on the connecting strand of chromatin); these leukocytes are motile phagocytes with distinctive antiparasitic functions F ferritin: an iron-protein complex, containing up to 23% iron, formed by the union of ferric ions with apoferritin; it is found in the intestinal mucosa, spleen, bone marrow, reticulocytes, and liver, and regulates iron storage and transport from the intestinal lumen to plasma folic acid: a collective term for pteroylglutamic acids and their oligoglutamic acid conjugates; a collective term for pteroylglutamic acids and their oligoglutamic acid conjugates; N-[p-[[(2-Amino-4-hydroxypteridin-6-yl)methyl]amino]benzoyl]-L(+)-glutamic acid; specifically, pteroylmonoglutamic acid; the growth factor for Lactobacillus casei, and a member of the vitamin B complex necessary for the normal production of red blood cells; present, with or without L(+)- glutamic acid moieties, in peptide linkages in liver, green vegetables, and yeast; used to treat folate deficiency and megaloblastic anemia G gamma glutamyl transferase (GGT): an enzyme that catalyzes the transfer of a γ-glutamyl group from a γ-glutamyl peptide to an acceptor gastroesophageal reflux disease (GERD): a regurgitation of the contents of the stomach into the esophagus, possibly into the pharynx where they can be aspirated between the vocal cords and down into the trachea; symptoms of burning pain and acid taste result; pulmonary complications of aspiration are dependent upon the amount, content, and acidity of the aspirate glomerular: relating to or affecting a glomerulus or the glomeruli 253 gluconeogenesis: the formation of glucose from noncarbohydrates, such as protein or fat glycogenolysis: the hydrolysis of glycogen to glucose glycosuria: urinary excretion of carbohydrates granular leukocytes: any one of the polymorphonuclear leukocytes, especially a neutrophilic leukocyte H hematocrit (HCT): percentage of the volume of a blood sample occupied by cells hemoglobin (HGB): the red respiratory protein of erythrocytes, consisting of approximately 3.8% heme and 96.2% globin, with a molecular weight of 64,450, which as oxyhemoglobin (HbO2) transports oxygen from the lungs to the tissues where the oxygen is readily released and HbO2 becomes Hb; when Hb is exposed to certain chemicals, its normal respiratory function is blocked; e.g., the oxygen in HbO2 is easily displaced by carbon monoxide, thereby resulting in the formation of fairly stable carboxyhemoglobin (HbCO), as in asphyxiation resulting from inhalation of exhaust fumes from gasoline engines; when the iron in Hb is oxidized from the ferrous to ferric state, as in poisoning with nitrates and certain other chemicals, a nonrespiratory compound, methemoglobin (MetHb), is formed; in humans there are at least five kinds of normal Hb: two embryonic Hb’s (Hb Gower-1, Hb Gower-2), fetal (Hb F), and two adult types (Hb A, Hb A2); there are two α globin chains containing 141 amino acid residues, and two of another kind (β, γ, δ, ε, or ζ), each containing 146 amino acid residues in four of the Hb’s; Hb Gower-1 has two ζ chains and two ε chains; the production of each kind of globin chain is controlled by a structural gene of similar Greek letter designation; normal individuals are homozygous for the normal allele at each locus; substitution of one amino acid for another in the polypeptide chain can occur at any codon in any of the five loci and have resulted in the production of many hundreds of abnormal Hb types, most of no known clinical significance; in addition, deletions of one or more amino acid residues are known, as well as gene rearrangements due to unequal crossing over between homologous chromosomes; the Hb types below are the main abnormal types known to be of clinical significance; newly discovered abnormal Hb types are first assigned a name, usually the location where discovered, and a molecular formula is added when determined; the formula consists of Greek letters to designate the basic chains, with subscript 2 if there are two identical chains; a superscript letter (A if normal for adult Hb, etc.) is added, or the superscript may designate the site of amino acid substitution (numbering amino acid residues from the N-terminus of the polypeptide) and specifying the change, using standard abbreviations for the amino acids; there is an exhaustive listing of variant hemoglobins in MIM where a composite numbering system is used 254 hemoglobin S disease: an abnormal Hb with substitution of valine for glutamic acid at the 6th position of the β chain; the formula is α2Aβ2S, or, more specifically, α2Aβ26Glu→Val; heterozygous state: sickle cell trait, no anemia, Hb S 20-45% of total, the rest Hb A; homozygous state: sickle cell anemia, Hb S 75-100% of total, the rest Hb F or Hb A2 hemoglobin S trait: heterozygous state in which there is one normal beta chain and one sickle beta chain, no clinical or hematological manifestations hemostasis: the arrest of bleeding hepatitis: inflammation of the liver, due usually to viral infection but sometimes to toxic agents hyperaldosteronism: a disorder caused by excessive secretion of aldosterone hyperglycemia: an abnormally high concentration of glucose in the circulating blood, seen especially in patients with diabetes mellitus hyperthyroidism: an abnormality of the thyroid gland in which secretion of thyroid hormone is usually increased and is no longer under regulatory control of hypothalamic-pituitary centers; characterized by a hypermetabolic state, usually with weight loss, tremulousness, elevated plasma levels of thyroxin and/or triiodothyronine, and sometimes exophthalmos; may progress to severe weakness, wasting, hyperpyrexia, and other manifestations of thyroid storm; often associated with exophthalmos (Grave’s disease) hyperthyroxinemia: an elevated thyroxine concentration in the blood hypoplastic anemia: progressive nonregenerative anemia resulting from greatly depressed, inadequately functioning bone marrow; as the process persists, aplastic anemia may occur hypothyroidism: diminished production of thyroid hormone, leading to clinical manifestations of thyroid insufficiency, including low metabolic rate, tendency to weight gain, somnolence and sometimes myxedema hypothyroxinemia: a subnormal thyroxine concentration in the blood I indirect bilirubin: the fraction of serum bilirubin which has not been conjugated with glucuronic acid in the liver cell; so called because it reacts with the Ehrlich diazo reagent only when alcohol is added; increased levels are found in hepatic disease and hemolytic conditions interindividual variation: deviation in true value of an analyte between individuals intraindividual variation: deviation in true value of an analyte within the same individual 255 iron: a metallic element, atomic no. 26, atomic wt. 55.847, that occurs in the heme of hemoglobin, myoglobin, transferrin, ferritin, and iron-containing porphyrins, and is an essential component of enzymes such as catalase, peroxidase, and the various cytochromes; its salts are used medicinally isoenzyme: one of a group of enzymes that catalyze the same reaction but may be differentiated by variations in physical properties, such as isoelectric point or modes of regulation isosthenuria: a state in chronic renal disease in which the kidney cannot form urine with a higher or a lower specific gravity than that of protein free plasma; specific gravity of the urine becomes fixed around 1.010, irrespective of the fluid intake K ketonemia: the presence of recognizable concentrations of ketone bodies in the plasma ketonuria: enhanced urinary excretion of ketone bodies L leukemia: progressive proliferation of abnormal leukocytes found in hemopoietic tissues, other organs, and usually in the blood in increased numbers; leukemia is classified by the dominant cell type, and by duration from onset to death; this occurs in acute leukemia within a few months in most cases, and is associated with acute symptoms including severe anemia, hemorrhages, and slight enlargement of lymph nodes or the spleen; the duration of chronic leukemia exceeds one year, with a gradual onset of symptoms of anemia or marked enlargement of spleen, liver, or lymph nodes leukocytes: a type of cell formed in the myelopoietic, lymphoid, and reticular portions of the reticuloendothelial system in various parts of the body, and normally present in those sites and in the circulating blood (rarely in other tissues); under various abnormal conditions, the total numbers or proportions, or both, may be characteristically increased, decreased, or not altered, and they may be present in other tissues and organs; leukocytes represent three lines of development from primitive elements: myeloid, lymphoid, and monocytic series; on the basis of features observed with various methods of staining with polychromatic dyes (e.g., Wright stain) cells of the myeloid series are frequently termed granular leukocytes, or granulocytes; cells of the lymphoid and monocytic series also have granules in the cytoplasm but, owing to their tiny, inconspicuous size and different properties (frequently not clearly visualized with routine methods), lymphocytes and monocytes are sometimes termed nongranular or agranular leukocytes; granulocytes are commonly known as polymorphonuclear leukocytes (also polynuclear or multinuclear leukocytes), inasmuch as the mature nucleus is divided into two to five rounded or ovoid lobes that are connected with thin strands or small bands of chromatin; they consist of three distinct types: neutrophils, eosinophils, and basophils, named on the basis of the staining reactions of the cytoplasmic granules 256 leukopenia: the antithesis of leukocytosis; any situation in which the total number of leukocytes in the circulating blood is less than normal, the lower limit of which is generally regarded as 4000-5000/mm3 lipase: in general, any fat-splitting or lipolytic enzyme; a carboxylesterase lipid: fat soluble, an operational term describing a solubility characteristic, not a chemical substance, i.e. denoting substances extracted from animal or vegetable cells by nonpolar solvents lipoprotein: any complex or compound containing both lipid and protein lymphocyte: a white blood cell formed in lymphatic tissue throughout the body (e.g., lymph nodes, spleen, thymus, tonsils, Peyer patches, and sometimes in bone marrow) and in normal adults making up approximately 22-28% of the total number of leukocytes in the circulating blood; lymphocytes are divided into 2 principal groups, termed T and B cells, based on their surface molecules as well as function lymphopenia: a reduction, relative or absolute, in the number of lymphocytes in the circulating blood M malignant: resistant to treatment; occurring in severe form, and frequently fatal; tending to become worse and leading to an ingravescent course mean: a statistical measurement of central tendency or average of set of values mean corpuscular hemoglobin (MCH): the hemoglobin content of the average red cell, calculated from the hemoglobin therein and the red cell count, in erythrocyte indices MCHC: mean corpuscular hemoglobin concentration (g/dL) MCV: mean corpuscular volume in femtoliters (fL) metabolic acidosis: a decreased pH and bicarbonate concentration in the body fluids caused either by the accumulation of acids or by abnormal losses of fixed base from the body, as in diarrhea or renal disease metabolic alkalosis: an alkalosis associated with an increased arterial plasma bicarbonate concentration, possibly resulting from an excessive intake of alkaline materials or an excessive loss of acid in the urine or through persistent vomiting; the base excess and standard bicarbonate are both elevated metastasis: the shifting of a disease or its local manifestations, from one part of the body to another 257 monoclonal gammopathy of undetermined significance (MGUS): a paraproteinemia (an abnormal gammaglobulin, typically with λ light chain component) of less than 3 g/100 ml, which at the time of discovery, is without apparent cause; specifically, there is no evidence of multiple myeloma or other malignant disorders monoclonal immunoglobulin: a homogeneous immunoglobulin resulting from the proliferation of a single clone of plasma cells and which, during electrophoresis of serum, appears as a narrow band or spike; it is characterized by heavy chains of a single class and subclass, and light chains of a single type monocyte: leukocytes of the circulating blood, normally found in lymph nodes, spleen, bone marrow, and loose connective tissue myoglobin: the oxygen-carrying and storage protein of muscle, resembling blood hemoglobin in function but containing only one subunit and one heme as part of the molecule, and with a molecular weight approximately one-quarter that of hemoglobin N negative predictive value: the fraction of negative values which are correct; determined by dividing the true negatives by the sum of the true negatives and false negatives neutropenia: the presence of abnormally small numbers of neutrophils in the circulating blood neutrophil: a mature white blood cell in the granulocytic series, formed by myelopoietic tissue of the bone marrow (sometimes also in extramedullary sites), and released into the circulating blood, where they normally represent 54-65% of the total number of leukocytes; the precursors of neutrophils, in order of increasing maturity, are: myeloblasts, promyelocytes, myelocytes, metamyelocytes, and band forms; although the terms neutrophilic leukocytes and neutrophilic granulocytes include younger cells in which neutrophilic granules are recognized, the two expressions are frequently used as synonyms for neutrophils, which are mature forms unless otherwise indicated by a modifying term, such as immature neutrophil; any cell or tissue that manifests no special affinity for acid or basic dyes, i.e. the cytoplasm stains approximately equally with either type of dye nongranular leukocytes: a general, nonspecific term frequently used with reference to lymphocytes, monocytes, and plasma cells; although the cytoplasm of a lymphocyte or monocyte contains tiny granules, it is nongranular in comparison with that of a neutrophil, basophil, or eosinophil O oliguria: scanty urine production osmol gap: the difference between the measured osmolality and the calculated osmolality 258 osmolality: the concentration of a solution expressed in osmoles of solute particles per kilogram of solute P pancytopenia: pronounced reduction in the number of erythrocytes, all types of white blood cells, and the blood platelets in the circulating blood paraprotein: a monoclonal immunoglobulin of blood plasma, observed electrophoretically as an intense band in γ, β, or α regions, due to an isolated increase in a single immunoglobulin type as a result of a clone of plasma cells arising from the abnormal rapid multiplication of a single cell; the finding of a paraprotein in a patient’s serum indicates the presence of a proliferating clone of immunoglobulin-producing cells and may be seen in a variety of malignant, benign, or non-neoplastic diseases pharmacogenetics: the study of genetically determined variations in responses to drugs in humans or in laboratory organisms positive predictive value: the fraction of positive values which are correct; determined by dividing the true positives by the sum of the true positives and false positives precision: the closeness of scatter in multiple measurements of the same quantity R red blood count (RBC): Calculation of the number of red blood cells (RBC) in a cubic millimeter of blood, by means of counting the cells in an accurate volume of diluted blood reference range: the inner 95% of values for a laboratory test as measured in a defined population; the subject population is typically disease free with regards to the test of interest respiratory acidosis: acidosis caused by retention of carbon dioxide; due to inadequate pulmonary ventilation or hypoventilation, with decrease in blood pH unless compensated by renal retention of bicarbonate respiratory alkalosis: alkalosis resulting from abnormal loss of CO2 produced by hyperventilation, either active or passive, with concomitant reduction in arterial plasma bicarbonate concentration S sarcoma: a connective tissue neoplasm, usually highly malignant, formed by proliferation of mesodermal cells sensitivity: the proportion of affected individuals who give a positive test result for the disease that the test is intended to reveal; (true positive results divided by total true positive and false negative results) 259 specificity: true negative results as a proportion of the total of true negative and false positive results standard deviation: statistical index of the degree of scatter from the central tendency, namely, of the variability within a distribution T T cell lymphocyte: a thymocyte-derived lymphocyte of immunologic importance that is long-lived (months to years) and is responsible for cell-mediated immunity; T lymphocytes form rosettes with sheep erythrocytes and, in the presence of transforming agents (mitogens), differentiate and divide; these cells have characteristic CD3 surface markers and may be further divided into subsets according to function, such as helper, cytotoxic etc. thalassemia: any of a group of inherited disorders of hemoglobin metabolism in which there is impaired synthesis of one or more of the polypeptide chains of globin; several genetic types exist, and the corresponding clinical picture may vary from barely detectable hematologic abnormality to severe and fatal anemia thrombocytopenia: a condition in which there is an abnormally small number of platelets in the circulating blood thromobocyte: platelet; an irregularly shaped disk-like cytoplasmic fragment of a megakaryocyte that is shed in the marrow sinus and subsequently found in the peripheral blood, where it functions in clotting; a platelet contains granules in the central part (granulomere) and, peripherally, clear protoplasm (hyalomere), but no definite nucleus; is about one-third to one-half the size of an erythrocyte; and contains no hemoglobin thyroditis: inflammation of the thyroid gland thyrotropin releasing hormone (TRH): abbreviation for thyrotropin-releasing hormone thyrotropin: a glycoprotein hormone produced by the anterior lobe of the hypophysis that stimulates the growth and function of the thyroid gland; it also is used as a diagnostic test to differentiate primary and secondary hypothyroidism thyroxine: the l-isomer is the active iodine compound existing normally in the thyroid gland and extracted there from in crystalline form for therapeutic use; also prepared synthetically; used for the relief of hypothyroidism, cretinism, and myxedema total iron binding capacity (TIBC): the capacity of iron-binding protein in serum (transferrin) to bind serum iron toxicology: the science of poisons, including their source, chemical composition, action, tests, and antidotes transferrin: a nonheme β1-globulin of the plasma, capable of associating reversibly with up to 1.25 μg of iron per gram, and acting therefore as an iron-transporting protein 260 triglycerides: glycerol esterified at each of its three hydroxyl groups by a fatty acid triiodothyronine: a thyroid hormone normally synthesized in smaller quantities than thyroxine; present in blood and thyroid gland and exerts the same biologic effects as thyroxine but, on a molecular basis, is more potent and the onset of its effect is more rapid tumor: any swelling or tumefaction thyroid stimulation hormone (TSH): see thyrotropin U urea: the chief end product of nitrogen metabolism in mammals, formed in the liver by means of the Krebs-Henseleit cycle and excreted in normal adult human urine in the amount of about 32 g a day V vitamin B12: generic descriptor for compounds exhibiting the biological activity of cyanocobalamin (cyanocob(III)alamin); the antianemia factor of liver extract that contains cobalt, a cyano group, and corrin in a cobamide structure; the physiologically active vitamin B12 coenzymes are methylcobalamin and deoxyadenosinecobalamin; a deficiency of vitamin B12 is often associated with certain methylmalonic acidurias Von Willebrand’s disease: a hemorrhagic diathesis characterized by tendency to bleed primarily from mucous membranes, prolonged bleeding time, normal platelet count, normal clot retraction, partial and variable deficiency of factor VIIIR, and possibly a morphologic defect of platelets; autosomal dominant inheritance with reduced penetrance and variable expressivity, caused by mutation in the von Willebrand factor gene (VWF) on 12p; type III von Willebrand’s disease is a more severe disorder with markedly reduced factor VIIIR levels; there is a recessive version of this disease [MIM*277480] which has the remarkable property that it represents a mutation at the same locus as the dominant form W white blood count (WBC): calculation of the number of white blood cells (WBC) in a cubic millimeter of blood, by means of counting the cells in an accurate volume of diluted blood Wilson’s disease: a disease caused by a defect in copper metabolism, leading to deposition of copper in liver, brain, kidney and other tissues; characterized by cirrhosis of the liver and degenerative changes in the brain Z Zollinger Ellison syndrome: a peptic ulceration with gastric hypersecretion and gastrinoma of the pancreas or duodenum, sometimes associated with familial multiple endocrine adenomatosis type 1 261 262