Supplementary Information Predictive supracolloidal helices from
advertisement

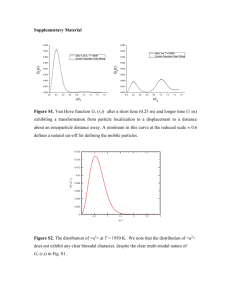
Supplementary Information Predictive supracolloidal helices from patchy particles Ruohai Guo, Jian Mao, Xu-Ming Xie and Li-Tang Yan * Key Laboratory of Advanced Materials (MOE), Department of Chemical Engineering, Tsinghua University, Beijing 100084, P.R. China [Content] 1. Details of the model and simulation methods 2. Design of patchy particles 3. Assembled structures obtained from various patchy particles 4. Measurement of the structural parameters of double helices 5. Characterization of the structural transition in the course of self-assembly 6. Observation of the reaction pathways 7. Theoretical modelling of circular dichroism of double helices 1 1. Details of the model and simulation methods We model the patchy character of a particle via an interaction potential that depends on both the separation between particle surfaces and the orientations of patch axes. The interaction potential, which was first proposed by Morgan et al.1, includes an isotropic component accounting for the short-range attraction and excluded volume of spherical particle and an anisotropic component describing the directional interaction between patches. In this model, two patchy particles, i and j , located at ri and r j respectively, feel an interaction given by U (i, j ) U M (rij ) U P (rij )(rij , pi )(rij , p j ) (1) pi , p j where rij ri r j , rij | rij | , and pi is the normalized vector pointing from the center of particle i towards its patch. In our simulations, three types of patches are used to decorate the patchy particles, that is, self-complementary patches marked as P and complementary patches marked as A or B. Patches P are set to interact only with other patches P, while patches A and B bind selectively only to each other. The isotropic component, U M , is a symmetric interaction and has the form of a 46-23 Mie potential U M (rij ) 4 rij 46 23 1 , 1 1/3 rij (2) where the parameter controls the range of the isotropic potential relative to the patch-patch interaction. For computational accuracy, U M is truncated and shifted to zero at rcutoff 2.5 . U P accounts for the attraction between the patches and is represented by a potential of tunable range and strength 2 r P 1 U P P [1 cos( (r ) s )] r s 1 2 r s 1 0 (3) where 1 (2 / ) 2/3 and the parameter s controls the slope within the range over which the attraction decays to zero. Finally, (rij , pi ) is a smooth function that modulates the angular dependence of the potential and is defined as 1 (cos cos ) 1 cos cos cos (rij , pi ) 2 1 cos 0 cos cos (4) 4 2 0 U -2 -4 -6 UM -8 UP (P = 10) -10 0.9 1.0 1.1 1.2 r 1.3 1.4 1.5 1.6 1.2 pi 1.0 pj 2 1 0.8 rij i j 0.6 0.4 0.2 0.0 0.0 Figure S1 | Illustration of the potential and its different components. Top, Plots of U M , U P for the parameter 40 , s 8 , and P 10 . Bottom, Sketch of the angular dependence of the interparticle potential as a function of their relative orientation. 3 Here, cos is the scalar product of the normalized vector rˆ ji with pˆ i . This particular form of patch-patch interaction is selected to generate a sufficiently smooth potential that is integrable in molecular dynamics. The parameters set in our model are 40 , s 8 and cos 0.94 (see Fig. S1 for an illustration of the potential). The areal coverage of a single patch is about 0.03. To determine the phase behavior for each type of patchy particle, Brownian dynamics simulations with a Langevin thermostat at constant temperature, T, are carried out. All the simulations are performed at a constant volume fraction of 0.0058 in a cubic box with periodic boundary conditions, and the number of particles N = 300 or 1000. The concentration is sufficiently small to reduce the possibility of formation of metastable, kinetically arrested structures. Particle position, velocities and angular velocities are updated through a velocity-Verlet scheme, where the particle orientations are updated using the equations for rotation of rigid bodies with quaternions2. Thermalization is achieved through application of a Langevin thermostat to both the linear and angular degrees of freedom. The particle mass is set by m, and the moment of inertial by I in accordance with a uniform density distribution within the particle. We calculate the interparticle forces and torques following the approach of Ref. 3. Briefly, the forces and torques are derived from the pair potentials between patchy particles and take the forms fi f j i pi j pj U U U U rˆij r 1 pˆ i pˆ j ˆ rˆ ) rij rij (pˆ j rˆij ) pi ,p j (p i ij (5) U (rˆij pˆ i ) (pˆ i rˆij ) (6) U (rˆij pˆ j ) (pˆ j rˆij ) (7) The notation pˆ pˆ (pˆ rˆ )rˆ rˆ (rˆ pˆ ) is short for the component of p̂ that is perpendicular to r̂ . 4 Each simulation runs for a minimum of 5 108 steps with a time step t 0.001. The natural units for these systems are the unit length, 0 , the bead mass, m , and 0 . The time scale is accordingly defined as 0 (m / 0 )1/2 . All quantities in this paper are expressed in dimensionless units. 2. Design of patchy particles The self-assembled helical structures are realized through rational design of patchy particles. The detailed parameters of patch directions and patch types are described in Table S1. Inspired by the sophisticated biological helices, we pattern particles’ surface with patches that mimic the interactions in biological macromolecules, including chemical bonds along the backbone of chains and hydrogen bonds in proteins or DNA molecules (Particle 1-3 in Table S1). Recent investigation1 provides another design of patchy particle that produces a compact three-stranded helix called Boerdijk-Coxeter (BC) helices or Bernal spirals (Particle 4 in Table S1). Considering the minimal model, we conclude that two types of interactions are required for cooperative, helical self-assembly: one interaction contributes to the stepwise growth of particle chains (P2 patches of particle 1-3); and the other interaction gives rise to large, helical, self-assembled structures (P1 patches of particle 1-3). Based on these rules, we induce a significantly simplified model of patchy particle that can also lead to helices with tunable structural metrics and handedness (Particle 5 in Table S1). Moreover, we notice that this strategy is analogous to the design of supramolecules that form tunable secondary structures. The patchy particles act as multifunctional monomer units that “react” with each other, in a process analogous to supramolecular polymerization. As shown in Fig. S2, the 5 intermolecular hydrogen bonds, π-π interactions, and metal-ligand binding are similar to patch interactions and govern the behavior of self-assembly. Table S1 | The patch direction and patch type for the particles shown in Fig. 1 and Fig. 2. The label P represents self-complementary patches while A and B is a pair of complementary patches. The θ and φ are the angles defined in the sketch of Fig. 2a. Particle number 1 2 3 4 5 Target structure Patch direction (xyz) Patch type (1, 0, 0) P1 (0.350, -0.477, -0.806) P2 (0.350, 0.477, 0.806) P2 (-0.8660, -0.5, 0) P1 (-0.8660, 0.5, 0) P1 (-0.098, -0.322, -0.942) P2 (-0.098, 0.322, 0.942) P2 (0, 0, 1) P1 (0, 0, -1) P1 (0.986, 0, 0.167) P2 (-0.439, 0.854, -0.285) P2 (-0.096, -0.301, -0.949) A (-0.577, 0.516, -0.633) A (-0.866, -0.387, -0.316) A (-0.866, 0.387, 0.316) B (-0.577, -0.516, 0.633) B (-0.096, 0.301, 0.949) B Double helices with (0, 0, 1) P tunable structures and (sin θ, 0, cos θ) A handedness (sin θ cos φ, sin θ sin φ, -cos θ) B DNA double-stranded helix triple-stranded DNA α-helix of proteins BC helices 6 a b Figure S2 | Molecular structures of supramolecules that are able to self-assemble into helical structures. a, C3-symmetrical bipyridine-based discotics. b, Bent-shaped bipyridine ligand containing a dendritic side chain and the silver complexes. The secondary structures of the complexes are dependent on the size of the counteranion. Inserts are cartoons representing their helical conformations. Figures reproduced with permission from: a, ref. 4, © 2002 ACS; b, ref. 5, © 2004 ACS. 7 3. Assembled structures obtained from various patchy particles Various helical structures are achieved through rational design of patchy particles. Figs. S3a, S3b and S3c show the helical structures that mimic chiral structure of biomolecules, including the DNA double helix, triple-stranded DNA, and α-helix of proteins. Fig. S3d also shows the snapshot of Boerdijk-coxeter (BC) helices, which have been found in colloidal clusters6 and have been investigated in computational models1. Fig. S4 shows the simulation snapshots of double helical structures with tailorable handedness and structural metrics. The helices shown in Fig. S4b have larger pitch and radius than the helices in Fig. S4a, and the helices shown in Fig. S4c have the same structural metrics and opposite handedness relative to the structures in Fig. S4a. The radial correlation functions g(r) of the helical structures shown in Fig. S4a and Fig. S4b are given in Fig. S6, in which the peaks of g(r) are consistent with the ideal helix models shown in the insets. Fig. S5 show typical configurations of patchy particles corresponding to various states included in the phase diagrams of Fig. 2e and Fig. 2f. 8 a b c d Figure S3 | Snapshots of helical structures obtained from patchy particles shown in Fig. 1. a, Double helices that mimic DNA double helix structure (εP = 20, T = 1.5). b, Triple helices analogous to triple-stranded DNA (εP = 20, T = 1.5). c, Single helices analogous to α-helix of proteins. The parameters set in a-c are (εP = 20, T = 1.5). d, Boerdijk-coxeter helices (εP = 10, T = 1.4, cos δ = 0.75). The simulations are performed on system size of N = 1000 for a-c and N = 300 for d. 9 a b c Figure S4 | Snapshots of double helices with tunable structural metrics and handedness obtained from the patchy particle shown in Fig. 2. a, Right-handed double helices from patchy particles with θ = 60° and φ = 120°. b, Right-handed double helices with large pitch and radius from patchy particles with θ = 60° and φ = 150°. c, Left-handed double helices with the similar structural metrics as a, where the patch parameters of patchy particle are θ = 60° and φ = -120°. All the simulations are performed on system size of N = 1000. 10 b a c d f e Figure S5 | Snapshots of configurations of patchy particles corresponding to different states in the phase diagrams shown in Fig. 2e and Fig. 2f. a, Arrested state with εP = 20 and T = 1.0. b, Liquid state with εP = 20 and T = 1.8. The patch parameters in a and b are θ = 60° and φ = 120°. c, Linear chains of patchy particles with θ = 45° and φ = 120°. d, Ring structures of patchy particles with θ = 80° and φ = 105°. The temperature in c and d is T = 1.2 and the strength of patch interaction εP = 20. e, A linear chain extracted from the structure shown in c. f, A ring extracted from the structure shown in d. 11 a 200 35 g(r) 25 g(r) 0σ 150 30 5σ 100 50 20 0 15 0 2 4 6 8 10 12 14 16 18 20 22 r/ 10 5 0 0 1 2 3 4 5 r/ b 7 8 9 10 200 35 0σ 150 g(r) 30 25 g(r) 6 5σ 100 50 20 0 15 0 2 4 6 8 10 12 14 16 18 20 22 r/ 10 5 0 0 1 2 3 4 5 r/ 6 7 8 9 10 Figure S6 | Characterization of helical structures. a, Radial correlation function of structure corresponding to Fig. 2b. b, Radial correlation function of structure corresponding to Fig. 2c. The values are averaged over 3 independent simulations and error bars indicate the standard deviation. Inserts show the models of ideal helix in which the patches interact and point perfectly at each other and the distance of nearest particles is set as the average patch bond length in simulations b0 = 1.16, corresponding to the minimum of the potential used. The color of particle in the models indicates the distance to the reference particle (white) based on the spectrum bar. 12 4. Measurement of the structural parameters of double helices To calculate the pitches and radii of helical structures assembled from various patchy particles, we examined several randomly selected locations along the backbone of helices. Fig. S7 shows the pitches and radii of the structures at various T and εP, corresponding to the phase diagram shown in Fig. 2e. Fig. S8 shows the pitches and radii of the structures obtained from patchy particles with different patch arrangements, corresponding to the phase diagram shown in Fig. 2f. 4.0 = 15 = 20 = 25 = 30 Theoretical pitch Theoretical radius 3.0 3.5 2.5 3.0 pitch 2.0 1.5 1.5 1.0 radius 2.0 2.5 1.0 0.5 0.5 0.0 0.0 1.0 1.2 1.4 1.6 1.8 T Figure S7 | The pitches and radii of patchy particle with θ =60°and φ =120° at various T and εP. The values are averaged over at least 10 locations along the backbone of helices: error bars indicate the standard deviation. The dashed lines are the pitch and radius of ideal helices. 13 a 8 = 105° = 120° = 135° = 150° pitch 6 4 2 0 50 55 60 65 70 75 80 (°) b 3.0 2.5 = 105° = 120° = 135° = 150° radius 2.0 1.5 1.0 0.5 0.0 50 55 60 65 70 75 80 (°) Figure S8 | The structural parameters of helices obtained from various patchy particles. a, The pitches of helices. b, The radii of helices. The error bars indicate standard deviation and the dashed lines indicate the theoretical values of pitches and radii of ideal helices. 14 5. Characterization of the structural transition in the course of self-assembly In order to characterize and distinguish the different assembled structures observed in the simulations, we have tracked the structural transition by matching the shape of assembled structure at a given time with the final helical structure. The shape matching algorithm7 is used to derive an order parameter S that distinguishes between randomly assembly and helical assembly. The general procedure for calculating the order parameter is as follows. First, the bond order diagram8 in the specific structures are constructed, the cutoff ranges are chosen as [0, 1.3σ], [1.3σ, 2.3σ] and [2.3σ, 3.3σ] corresponding to the three sharp picks in g(r) (Fig. S6a) to capture the different characteristic scales of helical structures. Second, the spherical harmonics coefficients of bond order diagrams are selected as shape descriptors. We choose the final helical structures as reference states and extract the shape descriptors. Subsequently, the shape descriptor of the reference state during self-assembly is determined and compared with that of the reference states to derive an order parameter: the closer the order parameter is to unity, the more the structure approaches the reference state. As shown in Figure S9, the matching order parameters with different characteristic scales present different dependence of time. The patchy particles interact directly and match well with the nearest particles at the preliminary stage, but the evolution of long-range ordered structure requires much longer time and involves complex mechanisms, as discussed in the main text. 15 b a d c 1.0 S 0.8 0.6 0 < rc < 1.3 0.4 1.3 < rc < 2.3 2.3 < rc < 3.3 0.2 0.0 0.5 1.0 t/ × 10 5 1.5 2.0 Figure S9 | Bond order diagrams and helical order parameters. a, Bond order diagram of helical structure within the cutoff range of [0, 1.3σ]. b, Bond order diagram within the cutoff range of [1.3σ, 1.3σ]. c, Bond order diagram within the cutoff range of [2.3σ, 3.3σ]. The surfaces of the bond order diagrams in a-c are smoothed for clarity by writing the density map as expansion in spherical harmonics and truncating the expansion at lmax = 16. d, Matching order parameters with different characteristic scales as functions of time. The values are averaged over 3 independent simulations and error bars indicate the standard deviation. Inserts show the typical structures extracted from snapshots at t 1104 , 5 104 and 1 105 , respectively. The parameters are T 1.2 , P 20 , and the patch direction 60 and 120 . 16 6. Observation of the reaction pathways To elucidate the kinetic pathways of self-assembly of helical structures, each cluster is monitored in the course of the self-assembly. This allows us to identify the structure evolution as shown in Fig. 4. Here, the details of snapshots of each reaction mechanism are displayed in Figs. S10-S12. All the simulations are performed at T 1.2 and P 20 , and the patch parameters of patchy particles are 60 and 120 . At the initial stage of self-assembly, the step-by-step addition of individual particles leads to the formation of small fragments. Subsequently, the small fragments add to each other and form a larger cluster. Both helical clusters (Fig. S10) and disordered clusters (Fig. S11b) can form because the addition of fragments is strongly affected by kinetics. The helical clusters can readily transform to large, perfect helix by slightly reconfiguration and addition (Fig. S11c). However the transformation of disordered clusters into thermodynamically favored helices is energetically more costly and would require much longer time because a number of patch bonds must be broken. Two types of mechanisms, i.e., fracture and reconfiguration, are observed in the transformation process of disordered clusters. In general, some bonds in the disordered cluster are broken due to thermal fluctuations or attack of other clusters. Thus the particles located at the defects have larger rotational freedom and it facilitates the patches to find their correct couples. Finally, the disordered cluster reconfigure to thermodynamically favored helices, as shown in Fig. S11d and Fig. S11e. In addition, we observed that some colloidal rings formed as a result of the cyclization of fragments (Fig. S11a) and fracture of disordered clusters (Fig. S11e). The colloidal rings are rather stable and do not substantially change during the simulation time. However, if a colloidal ring has some defects, it is unstable and will break at the location of defects (Fig. S12). 17 Figure S10 | Helical structure formed from small fragments. Reaction mechanisms of addition and fracture are denoted by blue arrows and purple dashed lines. Two fragments move close and form a larger cluster and the larger cluster then transform to a helix precursor and a complete helix by reconfiguration. 18 a b c d e Figure S11 | Examples of reaction mechanisms shown in Fig. 4. a, Formation of ring structure from small fragments. b, Disordered cluster formed from small fragments by addition. c, Larger helix formed from two smaller helices. d, Fracture of disordered cluster to a helix and a smaller disordered cluster. e, Fracture of disordered cluster to a ring and a helix. 19 a b c d e Figure S12 | Formation and break of incomplete ring structure. An incomplete ring is formed from a helical fragment. Because the ring is incomplete and unstable, it then breaks at the location of defect and adds to a pre-existing helix. 20 7. Theoretical modelling of circular dichroism of double helices Optical circular dichroism (CD) spectroscopy reveals chiral properties of molecules and assembly of particles. We assume Au nanoparticles with a diameter of 10 nm for patchy particle and the unit length σ0 = 10 nm to define the size of particle clusters. The CD response is computed using a numerical tool called DDSCAT 7.3.0, which was developed by Draine and Flatau 9. This numerical tool is based on the discrete dipole approximation (DDA) and is well known to reproduce essential spectral features of plasmonic nanostructures. In this approximation, a continuous medium is replaced by a set of N point dipoles defined on a cubic lattice. The equation for the dipole moment of the i-th dipole is Pi i (Eext,i E j i ) (8) j i where Eext,i is the external field, i is the polarizability of the i-th dipole, and the fields due to neighboring particles are 3r (r p ) p j (r p) rij ikrij E j i (1 ikrij ) ij ij 3 j k ij e rij rij (9) The polarizability i is defined following a modified version of the Clausius-Mossotti relationship that relates the macroscopic dielectric function of the metals to the microscopic polarizability of the point dipoles. For gold nanoparticles, we have used the data for the dielectric function from Johnson and Christy10. The problem to be solved can then be cast in the form AP E (10) where E is a 3N -dimensional complex vector of E ext at the N lattice sites, P is the unknown dipole polarizations, and A is a 3N 3N complex matrix. Then, the dipoles become iteratively computed from this system of equations. The extinction can be calculated using the following equation 21 1 Qext 0 Im(Eext,i* pi ) 2 i (11) where 0 is the dielectric constant of the matrix. Circular dichroism is defined as the difference in extinction of left-handed-circular polarized (LCP) and right-handed-circular polarized (RCP) light CD Q Q Since the nanostructures are randomly oriented in solution, the averaging over orientations, , is necessitated. We chose to fix the position and orientation of the helix and rotate the wave vector of the incident field, k , for convenience. The refractive index of matrix is n 1.333 (water). 22 a p = 30 nm r = 12.5 nm p = 40 nm r = 12.5 nm p = 50 nm r = 12.5 nm p = 40 nm r = 12.5 nm p = 40 nm r = 15.0 nm p = 40 nm r = 17.5 nm b c 8 2.0x10 p = 30 nm p = 40 nm p = 50 nm 8 CD (1/M cm) 1.5x10 8 1.0x10 7 5.0x10 0.0 7 -5.0x10 8 -1.0x10 8 -1.5x10 400 450 500 550 600 650 700 650 700 Wavelength (nm) d 8 2.0x10 r = 12.5 nm r = 15.0 nm r = 17.5 nm 8 CD (1/M cm) 1.5x10 8 1.0x10 7 5.0x10 0.0 7 -5.0x10 8 -1.0x10 8 -1.5x10 400 450 500 550 600 Wavelength (nm) Figure S13 | Circular dichroism of ideally helical structures. a, Representative sections of ideally helical structures with radii fixed at 12.5 nm and pitches p = 30 nm, 40 nm and 50 nm, respectively. b, Representative sections of ideally helical structures with pitches fixed at 40 nm and radii r = 12.5 nm, 15.0 nm and 17.5 nm, respectively. c. The theoretically predicted CDs for the geometries in a. d, The theoretically predicted CDs for geometries in b. 23 References 1. Morgan, J. W. R., Chakrabarti, D., Dorsaz, N. & Wales, D. J. Designing a Bernal Spiral from Patchy Colloids. ACS Nano 7, 1246-1256 (2013). 2. Miller, T. F. et al. Symplectic quaternion scheme for biophysical molecular dynamics. J. Chem. Phys. 116, 8649-8659 (2002). 3. Allen, M. P. & Germano, G. Expressions for forces and torques in molecular simulations using rigid bodies. Mol. Phys. 104, 3225-3235 (2006). 4. van Gorp, J. J., Vekemans, J. & Meijer, E. W. C3-symmetrical supramolecular architectures: Fibers and organic gels from discotic trisamides and trisureas. J. Am. Chem. Soc. 124, 14759-14769 (2002). 5. Kim, H. J., Zin, W. C. & Lee, M. Anion-directed self-assembly of coordination polymer into tunable secondary structure. J. Am. Chem. Soc. 126, 7009-7014 (2004). 6. Chen, Q. et al. Supracolloidal reaction kinetics of Janus spheres. Science 331, 199-202 (2011). 7. Keys, A. S., Iacovella, C. R. & Glotzer, S. C. Characterizing complex particle morphologies through shape matching: Descriptors, applications, and algorithms. J. Comput. Phys. 230, 6438-6463 (2011). 8. Roth, J. & Denton, A. R. Solid-phase structures of the Dzugutov pair potential. Phys. Rev. E 61, 6845-6857 (2000). 9. Draine, B. T. & Flatau, P. J. Discrete dipole approximation for scattering calculation. J. Opt. Soc. Am. A 11, 1491-1499 (1994). 10. Johnson, P. B. & Christy, R. W. Optical Constants of the noble metals. Phys. Rev. B 6, 4370-4379 (1972). 24