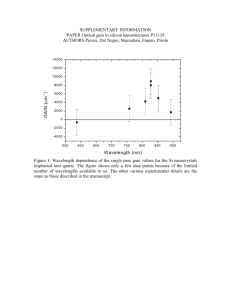
Nanowire-Aip advances-20120065-supp
advertisement

Fast vapor phase growth of SiO2 nanowires via surface-flow on Ag core/SiO2 shell structure Lei Gao1, Ailing Ji1, Nianpeng Lu1, Chaorong Li2 and Zexian Cao1* 1 Institute of Physics, Chinese Academy of Sciences, P. O. Box 603, Beijing 100190, China 2Department of Physics, Zhejiang Sci-Tech. University, Hangzhou 310018, China. E-mail: zxcao@iphy.ac.cn Supplemental material contents Figure S1: Correlation between the temperatures measured beneath the crucible and at the top of the substrate. Figures S2 and S3: SiO2 nanowires grown under various conditions. * Figure S4: Frozen Ag spherules sitting on SiO2 petals. Figure S5: SiO2 nanowires embedding pierced Ag particles. Figure S6: Catalysis of two SiO2 nanowires by one Ag particle. To whom correspondence should be addressed. E-mail: zxcao@iphy.ac.cn 1 Figure S1. Correlation between the temperature measured at the heat source beneath the crucible and that measured at the top of the substrate. This curve is helpful for the estimation of the temperature at the region concerned. Figure S2. SEM micrograph showing a part of substrate covered by interweaving SiO2 nanowires. Evaporation temperature: (1200℃). 2 Figure S3. In the region at the top of a substrate where it is relatively a little colder, aggregations of SiO2 particles, Ag crystals of micrometer size are visible through the thicket of nanowires. Evaporation temperature: 1100℃. Figure S4. SEM micrograph showing core/shell structures with the SiO2 shell just fully peeled off from the Ag core, resulting in Ag spherules sitting on a SiO2 pedestal. Some Ag spherules were removed, leaving behind the empty SiO2 pedestals. 3 Figure S5. SiO2 nanowires showing a chain of embedded Ag particles, resulting from the tearing of the catalytic Ag droplet in the case of abundant SiO2 molecules supply. Evaporation temperature: 1200℃. Figure S6. A Ag particle simultaneously catalyzing two nanowires of SiO2. This occurs when the primary liquid Ag core/ SiO2 shell structure comes into contact with the substrate at two points. 4