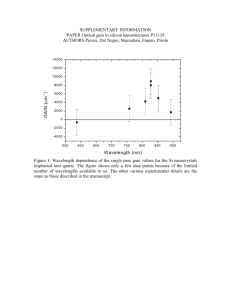
Thermal oxidation of Si:
advertisement

Thermal oxidation of Si: SiO2 – good electrical isolator Barrier to dopants Fick’s first law of diffusion: The particle flow per unit area J (particle flux) is directly proportional to the concentration gradient of the particles (N concentration of particles) D = diffusion coefficient Neg. sign = particles are moving from more concentrated to less concentrated area Assume: J is constant throughout the SiO2 volume – but is still a function of time. x = distance from surface of wafer No = concentration at the surface Ni = concentration at the interface between Si and SiO2 At Si-SiO2 interface assume that the oxidation rate is proportional to the concentration of the oxidant: ks = rate constant for the reaction at the Si-SiO2 inerface The rate of change of thickness of the oxide layer with time is given by the oxidizing flux J divided by the number of molecules of the oxidizing species that are incorporated into a unit volume (M) For thin layer time t is small so that B/A = linear (growth) rate constant Thin film limitation is caused by the reaction time in SiO2 B = parabolic rate constant