Introduction or preparing for the lab or background
advertisement

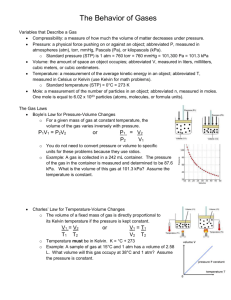
Adapted from Vernier Your Logo Pressure -Temperature Relationship in Gases Standards: 1.2.11.A Read and understand the central content of informational texts and documents in all academic areas. Use teacher and student established criteria for making decisions and drawing conclusions 3.2.12 B Evaluate experimental data correctly within experimental limits 3.4.10 A Predict the behavior of gases through the use of Boyle’s, Charles’ or the ideal gas law, in everyday situations. 3.7.10 C Apply knowledge of advanced input devices. Introduction and background: Gases are made up of molecules that are in constant motion and exert pressure when they collide with the walls of their container. The velocity and the number of collisions of these molecules are affected when the temperature of the gas increases or decreases. In this experiment, you will study the relationship between the temperature of a gas sample and the pressure it exerts. Using the apparatus shown in Figure 1, you will place an Erlenmeyer flask containing an air sample in water baths of varying temperature. Pressure will be monitored with a Gas Pressure Sensor and temperature will be monitored using a Temperature Probe. The volume of the gas sample and the number of molecules it contains will be kept constant. Pressure and temperature data pairs will be collected during the experiment and then analyzed. From the data and graph, you will determine what kind of mathematical relationship exists between the pressure and absolute temperature of a confined gas. You may also do the extension exercise and use your data to find a value for absolute zero on the Celsius temperature scale. Guiding questions: Based on everyday experiences, predict the relationship between the pressure and temperature of a confined gas at constant volume. Vocabulary: Absolute temperature is a temperature scale based on absolute zero. Same as Kelvin temperature scale. It is determined by adding 273 to the temperature value expressed in the centigrade scale. Pressure-Temperature Relationship In Gases Rev. 5/24/07 Page 1 Science In Motion Wilkes University Materials: computer 125 mL Erlenmeyer flask Vernier computer interface ring stand Logger Pro utility clamp Vernier Gas Pressure Sensor hot plate Vernier Temperature Probe four 800 mL beakers plastic tubing with two connectors glove or cloth rubber stopper assembly ice Safety: Wear safety glasses. Use caution when handling the hotplate and hot water. Do not allow the probe wires to touch the hotplate. Procedure: 1. Prepare a boiling-water bath. Put about 600 mL of hot tap water into an 800 mL beaker and place it on a hot plate. Turn the hot plate to a high setting. 2. Prepare an ice-water bath. Put about 500 mL of cold tap water into a second 800 mL beaker and add ice. 3. Put about 600 mL of room-temperature water into a third 800 mL beaker. 4. Put about 600 mL of hot tap water into a fourth 800 mL beaker. 5. Prepare the Temperature Probe and Gas Pressure Sensor for data collection. a. Plug the Gas Pressure Sensor into CH1 and the Temperature Probe into CH2 of the computer interface. b. Close the 2-way valve above the rubber stopper—do this by turning the valve handle so it is perpendicular with the valve stem itself (as shown in Figure 2). The air sample to be studied is now confined in the flask. Figure 2 6. Prepare the computer for data collection. a. Plug the Vernier Lab Pro interface into the power supply. Pressure-Temperature Relationship In Gases Page 2 Rev.5/24/06 Science In Motion Wilkes University b. Attach USB cable to Vernier Lab Pro interface and laptop computer. c. Power computer. d. Double click on the Logger Pro icon. e. Go to the File menu and select Open. f. Open “Chemistry with Computers.” g. Click on “07Pressure-Temperature.” 7. Click to begin data collection. 8. Collect pressure vs. temperature data for your gas sample: a. Place the flask into the ice-water bath. Make sure the entire flask is covered (see Figure 2). Stir. b. Place the temperature probe into the ice-water bath. c. When the pressure and temperature readings displayed in the meter stabilize, click . You have now saved the first pressure-temperature data pair. 9. Repeat the Step-8 procedure using the room-temperature bath. 10. Repeat the Step-8 procedure using the hot-water bath. 11. Use a ring stand and utility clamp to suspend the temperature probe in the boilingwater bath. To keep from burning your hand, hold the tubing of the flask using a glove or a cloth. After the temperature probe has been in the boiling water for a few seconds, place the flask into the boiling-water bath and repeat the Step-9 procedure. Remove the flask and the temperature probe after you have clicked . Click when you have finished collecting data. Turn off the hot plate. Record the pressure and temperature values in your data table, or, if directed by your instructor, print a copy of the table. 12. Examine your graph of pressure vs. temperature (°C). In order to determine if the relationship between pressure and temperature is direct or inverse, you must use an absolute temperature scale; that is, a temperature scale whose 0° point corresponds to absolute zero. We will use the Kelvin absolute temperature scale. Instead of manually adding 273 to each of the Celsius temperatures to obtain Kelvin values, you can create a new data column for Kelvin temperature. a. Choose New Calculated Column from the Data menu. b. Enter “Temp Kelvin” as the Name, “T Kelvin” as the Short Name, and “K” as the Unit. Enter the correct formula for the column into the Equation edit box. Type in “273+”. Then select “Temperature” from the Variables list. In the Equation edit box, you should now see displayed: 273+“Temperature”. Click . c. Click on the horizontal axis label and select “Temp Kelvin” to be displayed on the horizontal axis. 13. Decide if your graph of pressure vs. temperature (K) represents a direct or inverse relationship: a. If the points are not on the graph, single click on the Autoscale button from the menu. Pressure-Temperature Relationship In Gases Page 3 Rev.5/24/06 Science In Motion Wilkes University b. Click the Curve Fit button, . c. Choose your mathematical relationship from the list at the lower left. If you think the relationship is linear (or direct), use Linear. If you think the relationship represents a power, use Power. Click . d. A best-fit curve will be displayed on the graph. If you made the correct choice, the curve should match up well with the points. If the curve does not match up well, try a different mathematical function and click again. When the curve has a good fit with the data points, then click . e. Autoscale both axes from zero by double-clicking in the center of the graph toview Graph Options. Click the Axes Options tab, and from the scaling box select “Autoscale from 0” for both axes. 14. Print a copy of the graph of pressure vs. temperature (K). The regression line should still be displayed on the graph. Enter your name(s) and the number of copies you want to print Data table: Pressure (kPa) Temperature (°C) Temperature (K) Constant, k (P / T or P•T) Calculations: Computer performed calculations and graphing. Questions: 1. What two experimental factors (variables) must be kept constant during this lab? 2. Based on the data and graph that you obtained for this experiment, write a statement to express the relationship between gas pressure and temperature. 3. Explain this relationship using the concepts of molecular velocity and collisions of molecules. 4. Write an equation to express the relationship between pressure and temperature (K). Use the symbols P, T, and k. 5. One way to determine if a relationship is inverse or direct is to find a proportionality constant, k, from the data. If this relationship is direct, k = P/T. If it is inverse, k = P•T. Based on your answer to Question 4, choose one of these formulas and calculate k for the four ordered pairs in your data table (divide or multiply the P and T values). Pressure-Temperature Relationship In Gases Page 4 Rev.5/24/06 Science In Motion Wilkes University Show the answer in the fourth column of the Data and Calculations table. How “constant” were your values? 6. According to this experiment, what should happen to the pressure of a gas if the Kelvin temperature is doubled? Check this assumption by finding the pressure at – 73°C (200 K) and at 127°C (400 K) on your graph of pressure versus temperature. How do these two pressure values compare? References: Vernier ___________________________________ Pressure-Temperature Relationship In Gases Page 5 Rev.5/24/06 Science In Motion Wilkes University Pressure-Temperature Relationship in Gases Teacher notes Time needed to complete lab: This lab can be completed in a 50-60 minute period. Target grade level: This lab can be performed in a Chem I or Chem II class. Objectives: The objective is to determine the mathematical relationship between the pressure and temperature of a confined gas at a constant volume. Major concepts: This lab can be utilized when teaching gases. It must be emphasized that quantity and volume of the gas be kept constant. Preparation: Ice and warm water should be available. Also, it would be expedient to have the temperature probe, interface and the computer connected. Typical results or sample data: Sample calculations: Answers to questions: 1. The quantity and volume must be kept constant. 2. The pressure of a gas is directly proportional to the absolute temperature of the gas at constant quantity and volume. 3. As the temperature of the gas increases, the velocity of the molecules increases which results in an increase of molecular collisions with the surrounding walls. Thus the pressure increases. 4. P = k T 5. Utilizing k=P/T will produce an relatively constant value. 6. The pressure of the gas is doubled. Extension: The collected data can also be used to determine the value for absolute zero on the Celsius temperature scale. Instead of plotting pressure versus Kelvin temperature as done above, this time plot Celsius temperature on the y-axis and pressure on the xaxis. Since absolute zero is the temperature at which the pressure theoretically becomes equal to zero, the temperature where the regression line (the extension of the temperaturepressure curve) intercepts the y-axis should be the Celsius temperature value for absolute zero. 1. Remove the curve fit box on the graph by clicking on its upper-left corner. Pressure-Temperature Relationship In Gases Page 6 Rev.5/24/06 Science In Motion Wilkes University 2. Click on the vertical-axis label and select “Temperature” to plot the Celsius temperature. In the same way, select “Pressure” to be displayed on the horizontal axis. 3. If the points are not on the graph, single click on the Autoscale button from the menu. 4. Rescale the temperature axis from a minimum of –300°C to a maximum of 200°C. This may be done by clicking on the minimum and maximum values displayed on the graph axis and editing them. The pressure axis should be scaled from 0 kPa to 150 kPa. Rescaling can also be achieved by a right click on either axis followed by a left click on the “Graph Options.” Select the “Axes Options” tab. From the “scaling” boxes in the x-axis and y-axis locations select “manual” and type the range values. 5. Click the Linear Fit button, . A best-fit linear regression curve will be shown for the four data points. The equation for the regression line will be displayed in a box on the graph, in the form y = mx + b. The numerical value for b is the y-intercept and represents the Celsius value for absolute zero. 6. Print the graph of temperature (°C) vs. pressure, with the regression line and its regression statistics still displayed. Enter your name(s) and the number of copies you want to print. Clearly label the position and value of absolute zero on the printed graph. Pressure-Temperature Relationship In Gases Page 7 Rev.5/24/06 Science In Motion Wilkes University Science in Motion Wilkes University Pressure-Temperature Relationship in Gases Pre-Quiz 1. What is the relationship between pressure and temperature of a gas? a. b. c. d. 2. Predict how the pressure of a confined gas at the Earth’s surface will change if the confined gas is moved 100 miles above the Earth’s surface. a. b. c. d. 3. indirect direct not defined variable no change increase decrease not predictable When pressure-temperature data is graphed, the slope produced is a. b. c. d. negative positive zero undefined 4. In the study of the gaseous state, how many variables describe the state? a. b. c. d. one two four variable 5. Why can not the volume of gas change during the course of this experiment? a. b. c. d. It will affect the temperature. There will be no effect. The gas will explode. It will affect the pressure. Pressure-Temperature Relationship In Gases Page 8 Rev.5/24/06 Science In Motion Wilkes University Science in Motion Wilkes University Pressure-Temperature Relationship in Gases Post-Quiz 1. In the study of the gaseous state, how many variables describe the state? a. b. c. d. variable four two one 2. Why can not the volume of gas change during the course of this experiment? a. b. c. d. It will affect the pressure. The gas will explode. There will be no effect. It will effect the pressure. 3. Predict how the pressure of a confined gas at the Earth’s surface will change if the confined gas is moved 100 miles above the Earth’s surface. a. b. c. d. not predictable decrease increase no change 4. What is the relationship between pressure and temperature of a gas? a. b. c. d. variable not defined direct indirect 5. When pressure-temperature data is graphed, the slope produced is a. b. c. d. undefined zero positive negative Pressure-Temperature Relationship In Gases Page 9 Rev.5/24/06



![Temperature Notes [9/22/2015]](http://s3.studylib.net/store/data/006907012_1-3fc2d93efdacd086a05519765259a482-300x300.png)