Properties of Giant covalent molecules (macromolecules)
advertisement
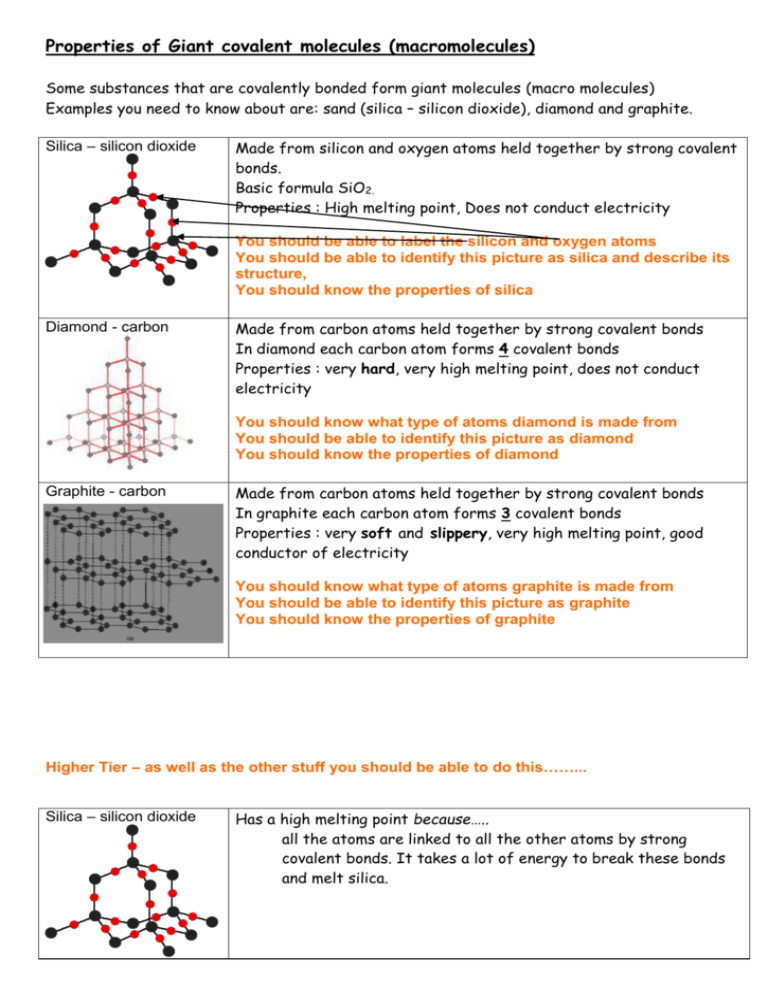
Properties of Giant covalent molecules (macromolecules) Some substances that are covalently bonded form giant molecules (macro molecules) Examples you need to know about are: sand (silica – silicon dioxide), diamond and graphite. Silica – silicon dioxide Made from silicon and oxygen atoms held together by strong covalent bonds. Basic formula SiO2. Properties : High melting point, Does not conduct electricity You should be able to label the silicon and oxygen atoms You should be able to identify this picture as silica and describe its structure, You should know the properties of silica Diamond - carbon Made from carbon atoms held together by strong covalent bonds In diamond each carbon atom forms 4 covalent bonds Properties : very hard, very high melting point, does not conduct electricity You should know what type of atoms diamond is made from You should be able to identify this picture as diamond You should know the properties of diamond Graphite - carbon Made from carbon atoms held together by strong covalent bonds In graphite each carbon atom forms 3 covalent bonds Properties : very soft and slippery, very high melting point, good conductor of electricity You should know what type of atoms graphite is made from You should be able to identify this picture as graphite You should know the properties of graphite Higher Tier – as well as the other stuff you should be able to do this……... Silica – silicon dioxide Has a high melting point because….. all the atoms are linked to all the other atoms by strong covalent bonds. It takes a lot of energy to break these bonds and melt silica. Diamond - carbon Has a high melting point because….. The carbon atoms are linked by 4 strong covalent bonds. It takes a lot of energy to break these bonds to melt diamond. Is hard because……. There are 4 very strong covalent bonds holding each atom of carbon to the others so they do not break off without a large force Graphite - carbon Does not conduct electricity because……. There are no free electrons to carry the current Has a high melting point because….. The carbon atoms are linked by 3 strong covalent bonds. It takes a lot of energy to break these bonds to melt graphite. Is soft and slippery because……. The 3 very strong covalent bonds hold the atoms of carbon in a layered structure. The 4th electron is delocalised (free to move) between the layers of carbon atoms and allows the layers to slide over each other very easily. Is a very good conductor of heat and electricity because……. The 4th electron from each carbon atom is delocalised (free to move) between the layers of carbon atoms and it flows to carry a current or moves around (diffuses) to transfer heat energy. You should be able to explain why silica, diamond and graphite have high melting points. You should be able to describe the structure of diamond You should be able to explain why diamond is very hard You should be able to describe the structure of graphite You should be able to explain why graphite is very soft You should be able to explain why graphite is a good conductor of electricity









