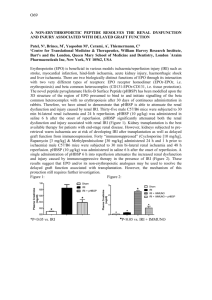
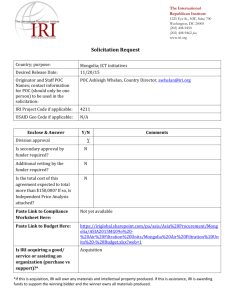
IgM Natural antibodies bind to the renal medulla in early ischaemia
advertisement

P213 INVOLVEMENT OF IGM NATURAL ANTIBODIES IN RENAL ISCHAEMIA REPERFUSION INJURY: A POTENTIAL NEW THERAPEUTIC TARGET? Ferenbach, D, Heelas, A, Kluth, D, Hughes, J Centre for Inflammation Research, Queens Medical Research Institute, University of Edinburgh INTRODUCTION: We have previously demonstrated that i.v. administration of apoptotic cells (AC) prior to renal ischaemia reperfusion injury (IRI) results in functional protection although the mechanism remains unclear. Constitutively expressed IgM natural antibodies (nAb) recognise conserved bacterial antigens but also bind to injured tissue. They are increasingly recognised to act as an innate arm of the adaptive immune system, with deposition of nAb shown to be important in mediating the initiation of mesenteric, skeletal and cardiac IRI. There is no data assessing nAb in renal IRI, however immunoglobulin deficient MT mice have intrinsic protection against IRI which is lost after adoptive transfer of serum but not lymphocytes. This work examined the primary hypothesis that nAb deposition could be present and important in renal IRI. Secondly, if nAb were bound by AC this could contribute to their protective effects in IRI. METHODS: IRI was induced in Balb/c mice and SCID mice (on the Balb/c genetic background) by 20min clamping of the left renal pedicle with right nephrectomy (n=810/group). Blood and kidneys were collected at 1hr and 24hrs post IRI. Murine thymocytes were rendered apoptotic ex vivo (>98% apoptotic) and 2x107 ACs injected i.v prior to experimental IRI. Biochemical and histological injury was assessed. Frozen sections were examined by immunofluorescence (IF) for IgM and C3 deposition. In vitro studies examined binding of IgM by ACs using flow cytometry with anti-IgM-APC Ab. RESULTS: IgM deficient SCID mice had preserved renal function compared to control Balb/c mice (serum Creatinine 94±16vs 140±14mol/L; p<0.05) after IRI. The kidneys of SCID mice showed structural protection, with less necrosis of outer medullary tubules (30±1% vs 56±4% necrotic tubules; p<0.01). 2x107 ACs injected 24h prior to IRI resulted in a 51% reduction in serum Creatinine (p<0.05) in Balb/c mice but had no protective effects in SCID mice, even when IRI was increased to 25min to augment injury levels. IF studies demonstrated that IgM is not present within the normal mouse medulla but is deposited in the first hour after IRI. This binding was not present in SCID kidneys. IgM was not detectable 24h after IRI indicating that it had either been cleared or the bound cells had died and been phagocytosed. In vitro studies demonstrated that serum from Balb/c mice contains nAb which bind to ACs, but SCID serum does not. CONCLUSION: These data demonstrate for the first time that nAb deposition is a feature of the immediate reperfusion phase of renal IRI. SCID mice lack nAb capable of recognising injured/dying cells in vitro and in vivo, whilst displaying a protected phenotype. Further studies are ongoing to characterise the effects of nAb repletion/depletion as a therapeutic strategy in IRI given the potential translational implications for both transplantation and acute kidney injury.