SP24 Apoptotic cell administration to mice modulates thrombin
advertisement

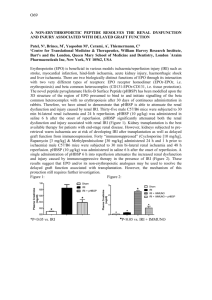
SP24 Apoptotic cell administration to mice modulates thrombin generation but is detrimental to renal function following renal ischaemia reperfusion injury Emily E. Hesketh1, Roger Preston2, David Kluth1 & Jeremy Hughes1 1 MRC Centre for Inflammation Research, Queen’s Medical Research Institute, University of Edinburgh. 2Department of Clinical Medicine, Trinity College Dublin Introduction: Acute kidney injury induced by renal ischaemia reperfusion injury (IRI) is characterised by microvascular congestion, acute inflammation and acute tubular necrosis (ATN). Apoptotic cells (ACs) administration reduces inflammation in various experimental models including LPS-mediated shock, collagen induced arthritis as well as lung and liver inflammation. Previous work in our lab suggested that intravenous administration of 20x106 ACs to FVB/n or Balb/c mice 24hrs prior to inducing renal IRI ameliorated renal function without affecting the ATN score 24hr following IRI. Objective: This study aimed to validate these early findings and explore if modulation of coagulation status leading to improved intrarenal microvascular flow might represent the underlying mechanism eliciting functional protection. Methods: ACs were generated from Balb/c thymocytes by overnight culture or incubation with dexamethasone. Annexin-V and propidium iodide (PI) staining was used to classify ACs as either early (Annexin-V+ PI-) or late (Annexin-V+ PI+) apoptotic by flow cytometry. AC modulation of coagulation was assessed by a thrombin generation assay on platelet-poor-plasma obtained 24hrs after mice received 20x106 early ACs. An in vitro assay was established to investigate whether ACs interacted with platelets. Platelets in whole blood were stained for the platelet surface integrin marker CD41 prior to incubation with ACs labeled with fluorescent cell tracker CM orange. Analysis was performed by flow cytometry. Renal IRI was modeled by a right nephrectomy (control kidney) and ischaemia induced by occlusion of the left renal pedicle in male Balb/c mice. Either 20x106 early or late ACs were administered 24hr prior to mild (22mins ischaemia), moderate (24 mins) or severe (25 mins) ischaemic injury. Renal function was assessed by plasma creatinine using a creatinase-based assay. Microvascular congestion was inferred from immunohistochemistry staining for CD41 and fibrin on frozen or paraffin embedded kidneys. Data expressed as mean ±SEM for PBS-treated vs. AC-treated mice. Results: The thrombin generation assay revealed that early AC-treated mice exhibited delayed thrombin generation as indicated by a significantly elevated lag time (2.1±0.2 vs. 3.2±0.3 mins, p<0.05) although the total amount of thrombin generated was similar to control mice (184±9.8 vs. 184±7.2 Thrombin nM). The in vitro analysis of ACs and platelet interaction confirmed ACplatelet binding as indicated by the presence of CM Orange+ ACs that were also CD41+. Despite the effect of ACs upon coagulation status, pretreatment of mice with ACs conferred no protection from renal IRI. Administration of early ACs did not preserve renal function from severe IRI (creatinine 99±9 vs. 107±15 µmol/L) nor influence fibrin and CD41 staining. In contrast, administration of early or late ACs significantly impaired renal function in mice with mild (creatinine 41±9 vs. 77±13 µmol/L, p<0.05) or moderate (creatinine 59±8 vs. 91±11 µmol/L, p<0.05) renal IRI, respectively. In addition, fibrin and CD41 accumulation was similar in PBS-treated and AC-treated mice following renal IRI. Conclusion: Unlike our preliminary data and despite demonstrable potential for AC administration to modulate coagulation pathways, and published data indicating AC modulation of inflammation and immunological pathways, administration of both early and late ACs was not protective in renal IRI. Depending upon the severity of ischaemic injury, administration of both early and late ACs may further impair renal function. This work contrasts with the ACderived protection observed in other organs and suggests that AC administration may not be translationally relevant for patients with acute kidney injury secondary to renal IRI.