Answers
advertisement

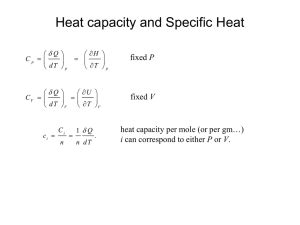
EAS 3603/6140: Thermodynamics of Atmospheres and Oceans Worksheet 5 – First Law of Thermodynamics 1. The first law of thermodynamics is a statement of the conservation of __Energy________ When an increment of heat dQ is added to a system, the energy may be used either to increase the speed of the molecules (i.e., to increase the temperature of the system), to create motion internal to each molecule (e.g., rotation and vibration), or to overcome the forces of attraction between the molecules (e.g., change of state from liquid to vapor), all of which contribute to the internal energy of the system. The internal energy of a system can increase when heat enters into the system from the surroundings, and/or when work is done on the system by the surroundings. From the statement of conservation of energy, we have (intensive form, using sign convention in book) du = dq + dw From the law of conservation of energy, the total energy of the system plus its environment must = syst + U env be constant. 0 U 2. What happens to internal energy in a cyclic process? (increase, decrease, remain the same) remains the same 3. Is internal energy an exact differential? YES NO Yes 4. It is convenient to define a new function called the enthalpy, h, by h = u + pv Expand the expression for h into a differential dh = . dh = du + d(pv) 5. Substitute for du the expression for the first law of thermodynamics in #1. dh = dq + vdp 6. Are the following equations both statements of 1st law of thermodynamics YES NO du = dq - pdv dh = dq + vdp Yes 7. Circle all of the following that are exact differentials: a) heat b) work c) internal energy d) enthalpy Explain why you think enthalpy is or is not an exact differential. Because all the functions used to calculate enthalpy are state functions 8. Write an expression for the internal energy of an ideal gas du = cvdT 9. Write an expression for the enthalpy of an ideal gas dh = cpdT 10. In a constant-pressure process, some of the added heat must be expended in doing work on the surroundings, while in a constant-volume process, all of the heat is devoted to raising the temperature of the substance. For the same material, how do the values of cp, cv compare? a) cp > cv b) cp < cv c) cp = cv 11. From the definition of enthalpy, h=u+pv, the differential form of enthalpy can be written as dh=du+d(pv). For an ideal gas, substitute values of dh, du, and d(pv) into this equation to derive a value for cp - cv for an ideal gas. NOTE: this expression is applicable only for an ideal gas. For non-ideal gases, liquids, and solids, values of cp, cv vary with temperature and pressure. dh du d ( pv) c p dT cv dT pdv vdp cv dT RdT c p cv R 12. If the value of cv for a monatomic gas is cv=(3/2)R, what is the value of cp? c p cv R c p 3 5 R R cp R 2 2 The following equations are both statements of 1st law of thermodynamics du = dq - pdv dh = dq + vdp For ideal gases, u and h have been shown experimentally to be functions only of T so that du = cv dT dh = cp dT 12. Write the 1st law in both internal energy and enthalpy forms for an ideal gas, combining the above expressions. cvdT = dq - pdv cpdT = dq + vdp Consider an ideal gas that is subject to expansion work. In the following, write expressions of the first law (starting from equations in #12), differential form with intensive variables, that are applicable ONLY to an ideal gas with expansion work 13. Write an expression for the first law of thermodynamics for constant volume process (note: do not write a general expression for the first law, but one that is applicable ONLY to constant volume processes). Is it more sensible to write this expression in terms of the internal energy or the enthalpy? du = dq – pdv dv=0 du = dq internal energy 14. Write an expression for the first law of thermodynamics for isobaric process (note: do not write a general expression for the first law, but one that is applicable ONLY to isobaric processes). Is it more sensible to write this expression in terms of the internal energy or the enthalpy? dh = dq + vdp dp=0 dh = dq enthalpy 15. Write an expression for the first law of thermodynamics for an isothermal process (note: do not write a general expression for the first law, but one that is applicable ONLY to isothermal processes). Is it more sensible to write this expression in terms of the internal energy or the enthalpy? cvdT = dq – pdv cvdT = 0 dq = pdv either would work 16. Write an expression for the first law of thermodynamics, enthalpy form, for an adiabatic process. (note: do not write a general expression for the first law, but one that is applicable ONLY to adiabatic processes) cpdT = dq + vdp dq = 0 cpdT = vdp 17. Starting from the expression you obtained in #16, substitute the ideal gas law for specific volume. You should now have and equation that contains only T, p as thermodynamic state variables. cpdT = RT/p dp 18. Integrate the expression you obtained in #17 from (T1, p1) to (T2, p2). T2 dT T R p2 dp R p T1 T c p p1 p ln T12 c p ln p12 19. Take anti-logs of the expression you obtained in #18. R T1 p2 c p T2 p1 By repeating #16-#19 but starting from the internal energy form of the first law, the following expression is derived T2 v = v1 T1 2 Rc v Substitituing from the ideal gas law and expression from #7, can also write relation between p, v p2 v = v1 p1 2 cp cv These expressions can also be written as Tvn-1 =const pvn =const Tp(1-n)/n =const where n is the polytropic index which for an ideal gas is n=cp/cv. For more general processes do not involve ideal gas and may not be adiabatic, the polytropic expressions are frequently used, but with an empirically-derived value of n. Dry Adiabatic Lapse Rate: 20. Write the first law of thermodynamics, enthalpy form, adiabatic, intensive, for an ideal gas (see #17). cpdT = vdp 21. Starting from #20, substitute the hydrostatic equation for the appropriate part of the expression in #20. dp g dz c p dT v gdz gdz (v 1) 22. Starting from #21, write an expression for the dry adiabatic lapse rate, d (note, if you did this correctly, you should obtain equation (2.68).) dT v g g d (v 1) dZ cp cp 23. What is the magnitude of the dry adiabatic lapse rate for the atmosphere? 9.8C / km 24. How does the magnitude of the dry adiabatic lapse rate compare with: a) the observed tropospheric lapse rate larger than the observed one b) the lapse rate of the homogeneous atmosphere (worksheet #2, section 1.10) in this case the dry rate is less