5th Grade Mixtures and Solutions
advertisement
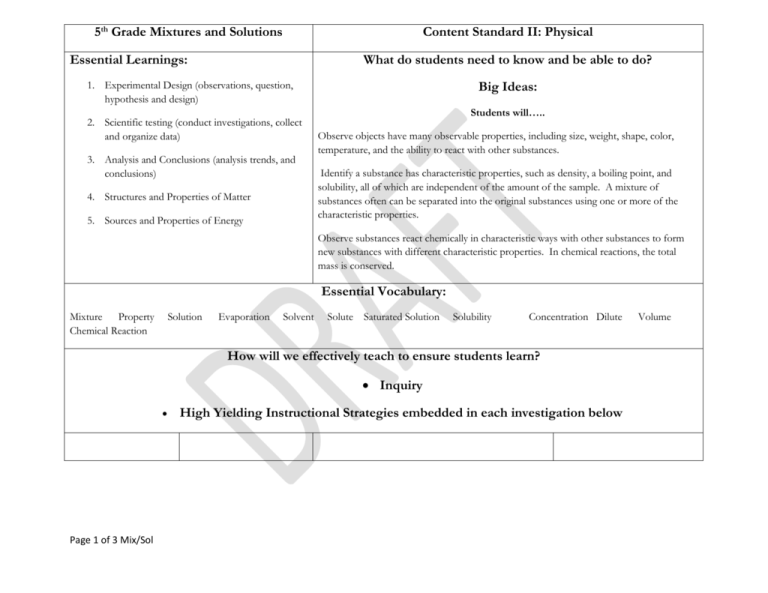
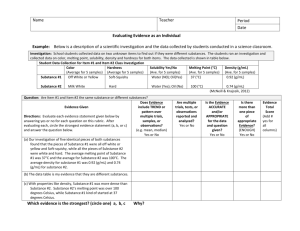
5th Grade Mixtures and Solutions Content Standard II: Physical Essential Learnings: What do students need to know and be able to do? Big Ideas: 1. Experimental Design (observations, question, hypothesis and design) 2. Scientific testing (conduct investigations, collect and organize data) 3. Analysis and Conclusions (analysis trends, and conclusions) 4. Structures and Properties of Matter 5. Sources and Properties of Energy Students will….. Observe objects have many observable properties, including size, weight, shape, color, temperature, and the ability to react with other substances. Identify a substance has characteristic properties, such as density, a boiling point, and solubility, all of which are independent of the amount of the sample. A mixture of substances often can be separated into the original substances using one or more of the characteristic properties. Observe substances react chemically in characteristic ways with other substances to form new substances with different characteristic properties. In chemical reactions, the total mass is conserved. Essential Vocabulary: Mixture Property Chemical Reaction Solution Evaporation Solvent Solute Saturated Solution Solubility Concentration Dilute How will we effectively teach to ensure students learn? Inquiry Page 1 of 3 Mix/Sol High Yielding Instructional Strategies embedded in each investigation below Volume Investigation 1: Separating Mixtures Essential Questions: How can a mixture be separated? How can a solution be separated? Investigation 2: Investigation 3: Concentration Reaching Saturation Investigation 4: Fizz Quiz Essential Questions: Essential Question: Essential Questions: Is there a limit to the amount of salt that can dissolve in 50 ml of water? How can an unknown chemical be identified by its solubility? How are materials identified by their crystals? What happens to the soft-drink solution when you increase the amount of powder in a given amount of water? How do you know a chemical reaction has occurred? How can you determine which of two salt solutions is more concentrated? How can you tell whether three solutions have different concentrations? What are the physical characteristics of solutions? How do you obtain a saturated solution using a variety of materials? Part 1: Making and Separating Mixtures Part 2: Separating a Salt Solution Part 3: Observing Crystals Part 4: Separating a Dry Mixture Page 2 of 3 Mix/Sol Part 1: Salt Saturation Part 1: Soft-Dink Part 1: Chemical Reactions Part 2: Citric-Acid Saturation Part 2: Salt Concentration Part 2: Reaction Products Part 3: The Saturation Puzzle Part 3: Reaction in a zip bag Part 4: Comparing Crystals Part 3: Mystery Solutions Part 4: Choosing your own Investigation What will we do if students don’t learn or if they are already performing at or above grade level? Science Extension opportunities provided in teachers manual Reteaching Strategies Suggested Time Frame: Scope and Sequence: 15 Investigations 45 minutes each DRAFT Page 3 of 3 Mix/Sol 5/8/2009