Mixtures & Solutions
advertisement

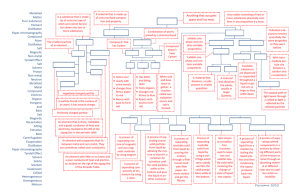
Mixtures & Solutions Investigation 1 Separating Mixtures Part 1 – Making & Separating Mixtures Vocabulary • Mixture – a substance containing two or more materials with different properties. • Property – a characteristic of an object (something you can observe) – Size, shape, color • Solution – a special mixture formed when a material dissolves in another. • Dissolving – a process in which one material disperses uniformly into another material so that the first material seems to disappear. – Two materials mixed evenly together Part 1 – Making & Separating Mixtures Main Ideas/Content • Water and solid material make a mixture. • Some mixtures can be separated by hand or with filters (ex: M & Ms, coins, water and gravel) • A mixture can be any combination of a solid, liquid, or gas. – Solid and solid – bronze (copper and tin), steel (carbon and iron) – Solid and liquid – vegetable soup, cereal & milk – Gas and gas – air – Gas and liquid – soda, seltzer water Part 2 – Separating a Salt Solution Vocabulary • Solvent – a substance that dissolves a solute to form a solution (ex: water). • Solute – a substance that dissolves in a solvent to form a solution (ex: salt, iced tea crystals, detergent). • Evaporate – to turn into a gas, like water into water vapor. • Element – a substance that cannot be broken down any further. • Atom – the smallest particle of an element. Atoms are the building blocks of matter. Part 2 – Separating a Salt Solution Main Ideas/Content • A solution is a special kind of mixture in which one of more materials dissolve in another (ex: salt water). – Solutions can be separated using evaporation • Almost everything on Earth is a mixture of two or more substances, except for elements. – There are 92 naturally occurring elements on Earth – Elements are arranged on the periodic table from lightest to heaviest. Part 3 – Observing Crystals Vocabulary • Crystal - the solid form of a material that can be identified by its properties, such as shape, color, and pattern. • Evaporation – causes liquids to dry up; the liquid turns into gas and disperses into the air. Part 3 – Observing Crystals Main Ideas/Content • When a solution evaporates, it leaves the dissolved solid material behind. • The salt crystal is a square with an X from corner to corner. • Salt is made up of the elements sodium (Na) and chlorine (Cl). – A substance made up of more than one element is a compound. Part 4 – Separating a Dry Mixture Main Ideas/Content (FOSS Science Stories – Earth Elements) • Humans are made up of 75% water. • Five elements make up most of the human body (and most other organisms) – Oxygen, carbon, hydrogen, nitrogen, calcium • Earth is made up of only a few main elements as well – Iron, oxygen, silicon, magnesium, nickel, sulfur • Although Earth and everything on it come from only a few elements, those elements combine in a huge variety of ways to create many different materials. Famous Scientists from Investigation 1 (From FOSS Science Stories) • Humphry Davy – discovered way to separate salt into sodium and chlorine. • Henry Cavendish – famous for his study of the composition of air; first person to isolate hydrogen from air. • Robert Boyle – “father of chemistry”; conducted many experiments with gases. • Marie Curie –discovered the elements radium and polonium; conducted research on radioactivity; won the Nobel prize twice.