Lab: Electron Cloud Model of the Atom
advertisement

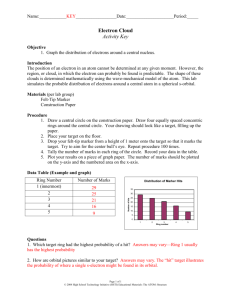
Name: __________________________________ Date: _________________ Period: ____ Lab: Electron Cloud Model of the Atom Introduction The position of an electron in an atom at a specific moment cannot be known. The regions of space in which electrons can probably be found are called orbitals or “electron clouds” because they are not exact. (in fact, electrons may sometimes even be found outside an orbital) The s orbital of hydrogen is represented by a fuzzy sphere with the nucleus at the center. The denser areas of sphere represent areas where there is a greater probability of finding hydrogen’s only electron. Since the electron is in constant motion, you can compare it to a spinning fan blade. The moving blade appears to fill the whole circle it spins through. In the same way, the single electron of a hydrogen atom appears to fill the entire electron cloud. Purpose In this lab, you will use a marker and a target to investigate the probability of finding marks (locations of electrons) around a central point (the nucleus). The marks are the chances of finding an electron in each area. This 2-D model will help you better understand the locations of electrons in the 3-D orbitals of atoms. Materials target marker meter stick Procedure 1. Tear off the back page with the target and place your target on a notebook on the floor. The target should be flat. The notebook simply serves as a cushion. 2. Drop your marker from a height of about 1 meter onto the target so that it makes a mark. Aim for the center. You should drop the marker 25 times, then let your partner drop 25 times. (TIP: Do not throw the marker. Let the marker fall from a position that appears to be directly over the center of the target.) 3. Count the number of marks in each numbered region of the target and record the numbers in the data table. (TIP: Circle the marks as you count them to avoid counting them twice.) Rules for counting marks: o If a mark is completely within a region, it belongs to that region. o If the mark is on a line, it belongs to the region that most of the mark lies in. o If the mark is on a line, and seems to be equally in both regions, it belongs to the region nearest the center (lowest number). 4. Return your marker. Clean any stray marks off the floor with a wet paper towel. 1 Data: Graph (Data Analysis): Region Number of Marks (# of times the e- was found) Probability of finding an e- in this region (# of marks times 2) 0 0 0 % (nucleus) 1 # of marks 2 3 4 5 6 7 (furthest from nucleus) Region Questions: 1. How does the probability of finding an electron change as you move outward further from the nucleus? ___________________________________________________ 2. How is your target with marks similar to the electron cloud of hydrogen’s only electron? _____________________________________________________________________ 3. What shape is an s orbital? ______________ 4. If you were to drop the marker one more time, in which region do you predict it would land? Region ____ Does your prediction mean it will definitely land there? ____ 5. Do your predictions mean that every mark will fall where you think? _____ Conclusion: Discuss the lab’s purpose (page 1) and what you observed and learned about the probable location of electrons in atoms including orbitals and electron clouds. 2 7 6 5 4 3 2 1 nucleus Count the number of marks in each numbered region of the target and record the numbers in the data table. (TIP: Circle the marks as you count them to avoid counting them twice.) Rules for counting marks: o If a mark is completely within a region, it belongs to that region. o If the mark is on a line, it belongs to the region that most of the mark lies in. o If the mark is on a line, and seems to be equally in both region, it belongs to the region nearest the center (lowest number). 3