Unit 4: Reactions & Stoichiometry Chapter Outline: Writing Chemical
advertisement

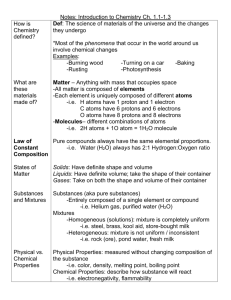
Unit 4: Reactions & Stoichiometry Chapter Outline: Writing Chemical Reactions (4.1) I. Chemical Reaction A. This is a process where one or more substances are changed into one or more different substances. 1. Reactants (sounds like “reaction”) a. These are the original substances that existed before the chemical reaction took place. b. These are usually found on the left side of a chemical reaction equation arrow. i. For reversible reactions, they could be on the right side…hence the term reversible. 2. Products (sounds like “produced”) a. These are the newly present substances that were produced after the reaction took place. b. These are usually found on the right side of a chemical reaction equation arrow. 3. Law of Conservation of Matter a. Matter is neither created nor destroyed… just transformed or transferred. b. The number of atoms, for each element in the equation, must be equal on both sides of the reaction arrow(s). i. This may require that you change coefficients (numbers in front of the chemical and usually referring to the number of moles present); not changing subscripts (as that would change the composition of the chemical.) For example: Ca(OH)2 + (NH4)2SO4 CaSO4 + 2NH3 + 2H2O Reactants Products Ca atoms = 1 Ca atoms = 1 N atoms = 2 N atoms = 2 S atoms = 1 S atoms = 1 H atoms = 10 H atoms = 10 O atoms = 6 O atoms = 6 II. Balancing Chemical Reactions A. Step 1: Identify the reactants and products for the chemical reaction. Step 2: Write the chemical reaction using the chemical formulas and appropriate symbols. Step 3: Count atoms & balance each element (one at a time) by changing coefficients; not the formula subscripts. * Start with elements other than Hydrogen or Oxygen. Leave those for last, as they will be present in several reactants or products possibly. Step 4: Double check by recounting elements (as seen in the above example) for your final reaction equation. III. Symbols often associated with chemical reactions: A. means “yields” B. means “reversible reaction” or “equilibrium” C. (s) – substance is in a solid state D. (l) – substance is in a liquid state E. (aq) – substance is aqueous (means “dissolved in water”) F. (g) – substance is in a gas state G. “Δ” or “heat” on top of the arrow – means reactants are heated H. “atm” on top of the arrow – means reactants are under pressure I. “0C” on top of the arrow – indicates the reaction temperature J. “ chemical name” on top of the arrow – indicates a chemical catalyst is present 1. Remember from Biology that a “catalyst” increases the rate of a chemical reaction without being consumed in the actual reaction. IV. Evidence for a chemical reaction occurring A. Presence of light being generated (like a match burning brightly.) B. A temperature change takes places within the reaction “chamber”. 1. Endothermic – absorbs heat energy from the environment (temperature decreases, feels cold) 2. Exothermic – releases heat energy to the environment (temperature increases, feels warm) C. Production of a gas. (Bubbles are seen or odor is detected.) D. Formation of a precipitate 1. This is the generated solid that may be associated with combining liquids. E. A color change takes places.