Biology Notes: Chemical Reactions & Enzymes
advertisement

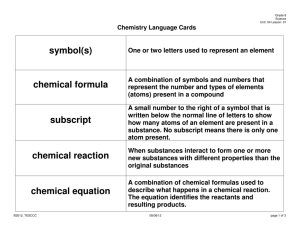
Biology Notes for Sect. 6.2 Main Idea: Chemical reactions allow living things to grow, develop, reproduce, and adapt. Objectives: 1. Identify the parts of a chemical reaction. 2. Relate energy changes to chemical reactions. 3. Summarize the importance of enzymes in living organisms. Chemical Reactions I. REACTANTS AND PRODUCTS a. Chemical Reaction-is the process by which atoms or groups of atoms in substances are reorganized into different substances. b. Clues that a chemical reaction has taken place include i. the production of heat or light, ii. and formation of a gas, liquid, or solid II. CHEMICAL EQUATIONS When scientists write chemical reactions, they express each component of the reaction in a chemical equation. a. Chemical Formulas- describe the substances in the reaction and arrows indicate the process of change. b. Reactants-are the starting substances, on the left side of the arrow. c. Products-are the substances formed during the reaction, on the right side of the arrow. d. Chemical Equation written to describe the reaction that provides energy: **Glucose and oxygen react to form carbon dioxide and water.** e. Balanced Equations i. Conservation of Mass-states matter cannot be created or destroyed. **This is IMPORTANT to REMEMBER because all Chemical Equations must show this balance of mass.** ii. The number of atoms of each element on the reactant side must equal the number of atoms of the same element on the product side. Notes for Balancing Equations: 1. Use coefficients to make the number of atoms on each side of the arrow equal. 2. Multiply the coefficient by the subscript for each element. III. ENERGY OF REACTIONS **The key to starting a chemical Reaction is energy.** Relation to Biology: most compounds in living things cannot undergo chemical reactions without energy. a. Activation Energy-is the minimum amount of energy needed for reactants to form products in a chemical reaction. b. Exothermic Reaction-the energy the product is lower than the energy of the reactants---it releases energy in the form of heat c. Endothermic Reaction-the energy of the products is higher than the energy of the reactant---it absorbs the heat energy IV. ENZYMES ***All living things are chemical factories driven by chemical reactions. However, these chemical reactions proceed very slowly when carried out in the laboratory because the activation energy needed is high. To be useful to living organisms, additional substances must be present where the chemical reactions occur to reduce the activation energy and allow the reaction to proceed quickly.*** a. Catalyst-is a substance that lowers the activation energy needed to start a chemical reaction. i. It does not increase how much product is made and it does not get used up in the reaction. b. Enzymes-are biological catalysts that speed up the rate of the chemical reactions in biological processes. i. Enzymes are essential to life ii. Enzymes’ names describe what they do. iii. Substrates-The reactants that bind to the enzyme iv. Active Site-The specific location where a substrate binds on an enzyme How it works: 1. The active site changes shape and forms the enzyme-substrate complex, which helps chemical bonds in the reactants to be broken and new bonds to form. 2. Factors such as pH, temperature, and other substances affect enzyme activity.