Chemistry Unit 7 Review: Chemical Reactions
advertisement

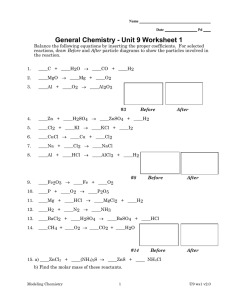
Name Date Pd Chemistry – Unit 7 Review: Chemical Reactions 1. Numbers placed in front of formulas in an equation are called 2. New substances formed in a chemical reaction are called 3. The total number of atoms represented by the formula Fe3(PO4)2 is 4. The symbol that indicates a substance dissolved in water is 5. Two white powders are mixed in a flask. The bottom of the flask feels cooler. This an example of what kind of reaction? 6. The charge on lead in PbSO4 is C B A B D D C + C A D + ? B 7. How many more D atoms should be on the right side of the equation? 8. What is the formula of the missing product molecule? 9. What are particles on the left side of the arrow called? 10. Write the completed chemical equation for the reaction pictured. Balance the following reactions. 11. ___C2H6 + ____Cl2 ––> ____CCl4 + ____HCl 12. ____Ca(OH)2 + ____FeCl3 ––> ____CaCl2 + ____Fe(OH)3 Modeling Chemistry 1 U7 Test-b-v2.0 Write balanced equations for each of the following chemical reactions. 13. Aluminum burns in air to form a grey solid, aluminum oxide. 14. Magnesium hydroxide reacts with hydrochloric acid (HCl) to form water and magnesium chloride. 15. The combustion of ethane gas, C2H6, produces carbon dioxide gas and water vapor. This reaction is exothermic. Include the energy term in your equation. Sketch the energy bar graph that represents the Eth and Ech and energy transfers at various stages in the reaction. 16. Below is a diagram showing the chemical energy (Ech) for the decomposition of water. Is the reaction exothermic or endothermic? Explain. 2 H + O2 2 en erg y 2 H2O OO O O 17. Describe the following reactions as combination, decomposition, single replacement, double replacement or combustion. a. 2 Mg + CO2 C + 2 MgO b. N2 + 3 Cl2 2 NCl3 c. C2H4 + 3 O2 –> 2 CO2 + 2 H2O Modeling Chemistry 2 U7 Test-b-v2.0