Josh Abecassis
advertisement

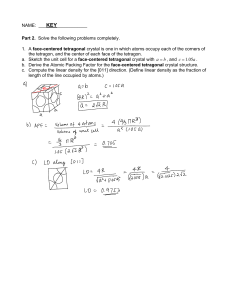
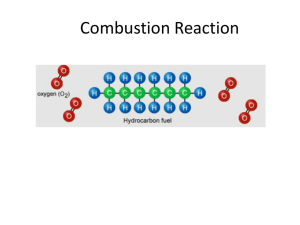
Josh Abecassis Josh Stone Thermodynamics Test 1. The Combustion of C14H30(l) is spontaneous at 25oC a. Write a balanced equation for this process b. Use the given bond energies to calculate the ∆H for the reaction (CC: 694 kJ, C-H:828 kJ, O=O: 498 kJ, O=C: 740 kJ, O-H: 464 kJ) c. What is the sign of the change in entropy? Explain qualitatively d. Suppose that the specific heats for this substance as a liquid and gas are 1.42 J/g* oC and 2.43 J/g* oC respectively. Also assume that the boiling point of the substance is 92oC and that the heat of vaporization is 2.98 J/g i. How much energy would be required to heat 1500 g of this substance from 54oC to 87oC? ii. How much energy would be required to heat 1500 g of this substance from 54oC to 100oC? 2. An unknown mass of liquid Nickel at 1700oC is dropped into 1.00 L of water at 25oC. The final temperature of the system is 48oC. Given that the specific heat for water as a liquid is 4.18J/g* oC, the specific heat for Nickel as a liquid is .732 J/g* oC, the specific heat for Nickel as a solid is .444 J/g* oC, the heat of fusion for nickel is 297.1 J/g, and that the melting point for Nickel is 1453 oC, determine the mass of the sample 3. Methane is the most basic organic molecule a. Write an equation for the combustion of methane b. Find the change in entropy based on the following given values (SCH4 = 186.3 J/mol, SO2 = 205.1 J/mol, SH2O = 188.8 J/mol, SCO2 = 213.7 J/mol) c. If ∆H for the reaction is -70.9 kJ/mol at 25 oC, find ∆G d. Find the equilibrium constant for the combustion of methane at 25 o C