ADVANCED POlYMER CHEMISTRY
advertisement
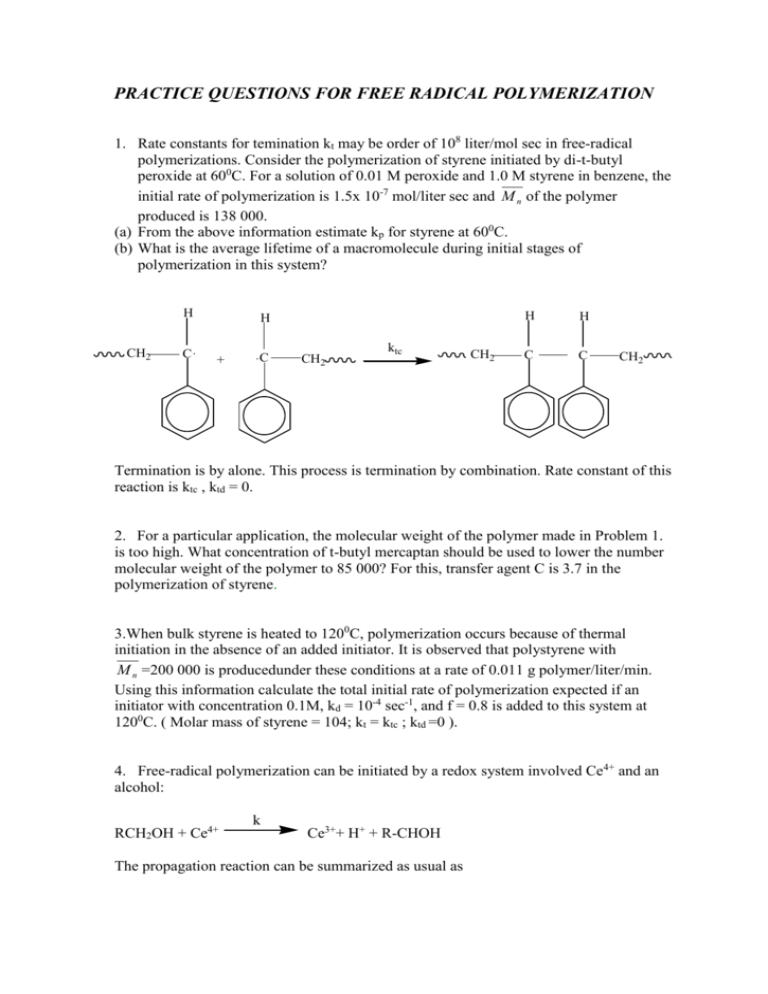
PRACTICE QUESTIONS FOR FREE RADICAL POLYMERIZATION 1. Rate constants for temination kt may be order of 108 liter/mol sec in free-radical polymerizations. Consider the polymerization of styrene initiated by di-t-butyl peroxide at 600C. For a solution of 0.01 M peroxide and 1.0 M styrene in benzene, the initial rate of polymerization is 1.5x 10-7 mol/liter sec and M n of the polymer produced is 138 000. (a) From the above information estimate kp for styrene at 600C. (b) What is the average lifetime of a macromolecule during initial stages of polymerization in this system? H CH2 C. H + .C CH2 ktc CH2 H H C C CH2 Termination is by alone. This process is termination by combination. Rate constant of this reaction is ktc , ktd = 0. 2. For a particular application, the molecular weight of the polymer made in Problem 1. is too high. What concentration of t-butyl mercaptan should be used to lower the number molecular weight of the polymer to 85 000? For this, transfer agent C is 3.7 in the polymerization of styrene. 3.When bulk styrene is heated to 1200C, polymerization occurs because of thermal initiation in the absence of an added initiator. It is observed that polystyrene with M n =200 000 is producedunder these conditions at a rate of 0.011 g polymer/liter/min. Using this information calculate the total initial rate of polymerization expected if an initiator with concentration 0.1M, kd = 10-4 sec-1, and f = 0.8 is added to this system at 1200C. ( Molar mass of styrene = 104; kt = ktc ; ktd =0 ). 4. Free-radical polymerization can be initiated by a redox system involved Ce4+ and an alcohol: RCH2OH + Ce4+ k Ce3++ H+ + R-CHOH The propagation reaction can be summarized as usual as kp Mn . + M Mn+1 Where Mn. is a macroradical with degree of polymerization n. The major termination reaction involves the Ce4+ component of the redox system. kt Mn . + Ce4++ Ce3++ H+ + dead polymer. Using the steady-state assumption, derive a useful expression for the rate of polymerization. Take the initiator efficiency = f and assume that the propagation reaction is the only one which uses significant quantities of monomer. 5. For acrylamide, kp2/kt = 22 liter/ mol sec at 250C and termination is by coupling alone. At this temperature the half-life of isobutylryl peroxide is 9.0h and its efficiency in methanol can be taken to equal to 0.3 A solution of 100 g/liter acrylamide in methanol is polymerized with 10-1 M isobutyryl peroxide. (a) what is the initial steady-state rate of polymerization? (b) How much polymer has been made in the first 10 min of reaction in 1 liter of solution? 6. One hundred liter of methyl methacrylate is reacted with 10.2 mol of an initiator at 600C. (a) What is the kinetic chain length in this polymerization? (b) How much polymer has been made in the first 5 h of reaction? kp= 5.5 liter/mol sec kt= 25.5 x 106 liter/ mol sec Density of monomer = 0.94 g cm3 t1/2 for this initiator= 50 h f = 0.3 100 L methyl methacrylate ( Mo )= C5H8O2 = 100 g/mol) 10.2 moles of an initiator at 60oC. 7. Redox initiation is often used in polymerization in aqueous system. Thus H2O2 and Fe2+ ion can be used to initiate the polymerization of acrylamide in water. Derive an expresiion for the steady-state rate of polymerization. In this case: H2O2 + Fe2+ k HO. + OH- + Fe3+ 8. (a) In a free-radical polymerization in solution the initial monomer concentration is increased by a factor of 10. (i) How is the rate of polmerization affected quantitatively? (ii) What change takes place in the number average degree of polymerization? (b) If the monomer concentration was not changed but the initiator concentration was halved instead: (i) What is the change in the rate of polymerization. (ii)What is the change in the number of average degree of polymerization? 9. Consider the chain transfer reaction that occur in the radical-initiated homopolymerizations of isopropenyl acetate and methacrylonitrile. Which monomer yields high-molecular-weight polymers is conventional free radical polymerizations? Explain the difference. 10. Vinyl acetate was polymerized in a free-radical reaction. The initial monomer concentration was 1 mol/ liter and is concentration after 1 h was 0.85 mol/liter. Chloroform was present as chain transfer agent,with concentrations 0.01 mol liter at time zero and 0.007 mol liter after 1h. What is the chain transfer constant C in this case? 11. An engineer is studying the azodiisobutyronile-initiated polymerization of butyl acrylate in solution. She wants to change to monomer and initiator concentrations so as to double the initial steady-state rate of polymerization without changing the number average degree of polymerization of the polymer or the reaction temperature. How should she proceed?