Polyaddition
advertisement
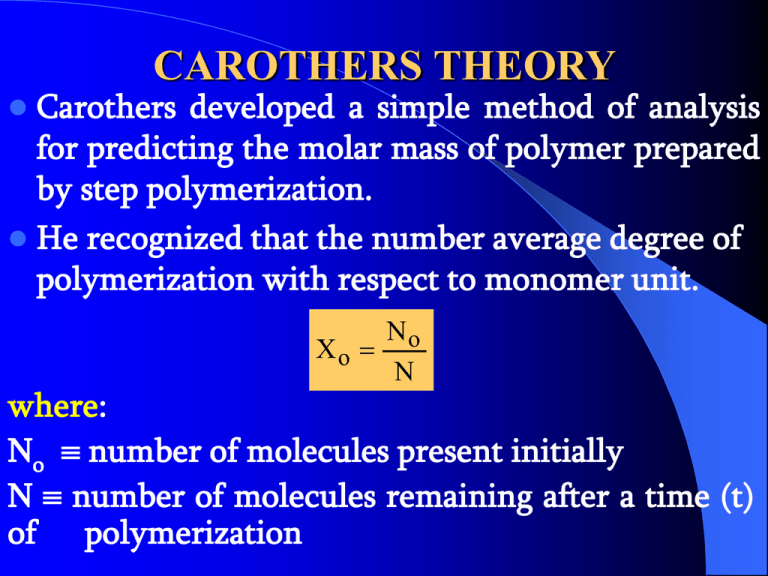
CAROTHERS THEORY Carothers developed a simple method of analysis for predicting the molar mass of polymer prepared by step polymerization. He recognized that the number average degree of polymerization with respect to monomer unit. No Xo N where: No number of molecules present initially N number of molecules remaining after a time (t) of polymerization ASSUMPTION FOR CAROTHERS THEORY There are equal numbers of functional groups, Xn can be related to the extent of reaction P at time t. Number of functional groups that have reacted P = _________________________________________________________ 1.13 Number of functional groups present initially He also assume that the probability that any functional group present initially has reacted. Therefore the total number of molecules decreases by one for each pair-wise reaction between functional groups. No N N N P , P 1 , P 1 No No No N No 1 1 P , No N 1 P Combining equation (1.12) and (1.13) gives the Carothers equation. 1 Xn 1 P This equation is applicable to RA2 + RB2 , ARB and RA2 polymerization’s in which there is an exact stoiciometric balance in the number of mutually reactive functional groups. KINETICS OF STEP POLYMERIZATION REACTION First we assume that the functional groups have equal reactivity. So we can apply a single rate constant for the step-wise reactions. Rate of reaction (overall) is defined as the rate of decrease in the concentration of one or other of the functional groups. i.e. dA dB Rate of reaction Where: dt dt A & B are the reactive group. Usually in step polymerization a catalyst is used. A B cat AB cat The rate of theforward reaction [A][B][cat ] dA dt Where: K’ is the rate constant of the reaction. Since the concentration of the catalyst does not change as the reaction proceed. ' [cat ] dA [A][ B] dt when [A] = [B] = C at equal molar stoiciometry. dC C 2 dt where C Co at t o Integrate c t dc c 2 co dt to Co No C N 1 1 1 t C Co N C Co No 1 t Co 1 No ( 1) t Co N No 1 Co t N N 1 P No N Co No 1 1 C o t 1 P This equation also applies to reaction which proceed in the absence of catalyst. Certain step polymerization are self-catalyzed i.e. one of the types of functional group acts as a cat. (E.g. carboxylic acid groups in a polyesterization). dA [A][ B][ A] dt if [A] = [B] = C dC 3 C dt if we integrate c t dc co or in c 3 dt 1 C to 2 1 Co 2 2t term of the extent of reaction 1 1 P2 1 2Co2 t These two equation derived assuming the depolymerization is negligible. This assumption is approach by in polycondensation reaction by the elimination of the product as it form. Normally the date are presented in a plot in the following form (a) (b) 1 1 _____ ________ 1 (1-P)2 -P t t Step polymerization of Polymerization of decamethylene glycol ethylene glycol with with adipic acid using adipic acid. toluene sulphonic acid as a catalyst. Thank You See You Next Lecture


![CHEE_392_-_Words_to_know-Processing[1] - P](http://s3.studylib.net/store/data/009652514_1-54ca9a81dd105bea22c19783eb204240-300x300.png)