Electric Nerves Class activity + background
advertisement
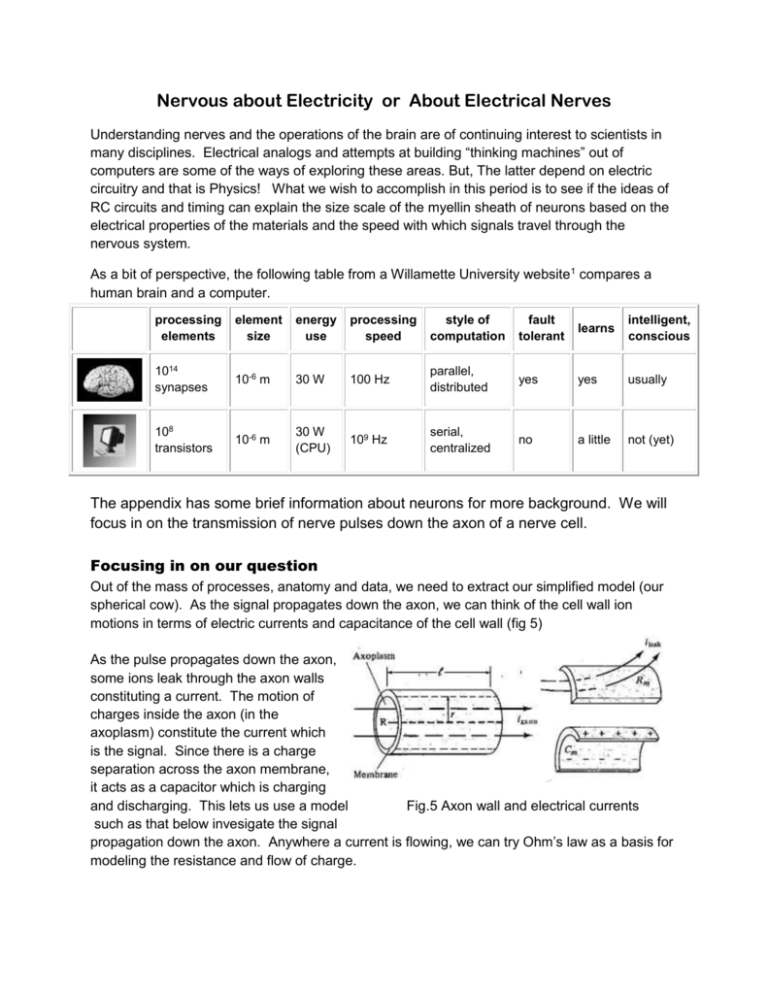
Nervous about Electricity or About Electrical Nerves Understanding nerves and the operations of the brain are of continuing interest to scientists in many disciplines. Electrical analogs and attempts at building “thinking machines” out of computers are some of the ways of exploring these areas. But, The latter depend on electric circuitry and that is Physics! What we wish to accomplish in this period is to see if the ideas of RC circuits and timing can explain the size scale of the myellin sheath of neurons based on the electrical properties of the materials and the speed with which signals travel through the nervous system. As a bit of perspective, the following table from a Willamette University website1 compares a human brain and a computer. processing elements element size energy use processing speed style of computation fault tolerant learns intelligent, conscious 1014 synapses 10-6 m 30 W 100 Hz parallel, distributed yes yes usually 108 transistors 10-6 m 30 W (CPU) 109 Hz serial, centralized no a little not (yet) The appendix has some brief information about neurons for more background. We will focus in on the transmission of nerve pulses down the axon of a nerve cell. Focusing in on our question Out of the mass of processes, anatomy and data, we need to extract our simplified model (our spherical cow). As the signal propagates down the axon, we can think of the cell wall ion motions in terms of electric currents and capacitance of the cell wall (fig 5) As the pulse propagates down the axon, some ions leak through the axon walls constituting a current. The motion of charges inside the axon (in the axoplasm) constitute the current which is the signal. Since there is a charge separation across the axon membrane, it acts as a capacitor which is charging and discharging. This lets us use a model Fig.5 Axon wall and electrical currents such as that below invesigate the signal propagation down the axon. Anywhere a current is flowing, we can try Ohm’s law as a basis for modeling the resistance and flow of charge. Modeling the axon as a circuit From the membrane potential, rate of ion flow and the surface charge and geometry of the axon walls, the following parameters are determined: Typical values for Axons: Quantity Axoplasm resistivity , ρa Capacitance/Area of membrane, Cm Resistance of unit Area of membrane, Rm Myelinated 2 Ω·m 5E-5 F/m2 40 Ω·m2 Typical dimensions: Axon radius Schwann cell width Unmyelinated 2 Ω·m 1E-2 F/m2 0.2 Ω·m2 5 μm 1 mm Node of Renvier width 1 μm Resistance for ion flow in axoplasm: Calculate the resistance in Ohms/cm using R = ρaℓ/A and the data above. For comparison, R for 70Mm of #40 Cu wire is about 3 x 108 Ω as is the resistance of 2.7 km of Si with a ceoss section of 0.25cm2. Using C = Cm 2π r ℓ [1] Calculate the values for the capacitance of 1 cm of myelinated axon : and 1 cm of unmyelinated axon : [2] Thinking about how the capacitance of a parallel plate capacitor is related to its structure, why is the capacitance per length of myelinated nerve lower than that for the unmyelinated one? As the charges flow inside the axon, we have a new situation: Charge flows along the circuit path (axon), but it may also leak out the sides (which was not possible with our wires in the last unit). There is a certain length at which the electrical resistance along the axon (the axoplasm resistance) is equal to the leakage resistance through the walls. [3] The resistance to flow through the axoplasm is what you calculated above. Determine the leakage resistance per cm for the unmyelinated axon and the myelinated axon using Rm from the table and the area per length ℓ is A=2πrℓ. [4] Find the length ℓ at which Rleak = Rplasma for both myelinated and unmyelinated axons. This is the length at which more signal is lost to leakage than flows down the axon. After the characteristic length ℓ calculated above, the signal must be amplified, which costs energy. Timing We have capacitance and resistance values for the axon wall, let’s see what we get for an RC time constant for discharging or recharging the capacitor charge layers along the membrane.To compare, the following figure gives typical times for the signal propagation. [5] Using the Rm and Cm values in the table, determine the RC time constant for changing the potential across the nerve membrane. Is the value different for myelinated and unmyelinated nerves? If you compare this with the timescale of the axon potential curve, you see it is in the right ballpark, but still slow by a bit less than an order of magnitude. This is because leakage through the membrane is only one way charge is transferred. The ion channels allowing active transport when opened play a huge role here in transferring ions more quickly across the membrane. This is, however, useful for analyzing the signal speed along an axon. This speed is equal to X/T where X is the distance between signal refresh (between nodes of Ranvier) and T is the RC time constant (to within a factor of 3 or so). The appropriate resistance to use here is the internal resistance Ra, since almost all of the charge flow is along the axon in the myelinated section between the nodes of Ranvier. The nodes are on the order of 1mm apart in a myelinated cell and using RC = RaC should be as good as the data, because there are no ion channels between the nodes. [6] Calculate the signal velocity = 1mm/RC for the myelinated axon. Values for the squid giant axon (1mm diameter, unmyelinated) have been reported to be around 25 m/s, how does your number compare and why? Modeling the axon as a circuit The axon can be modeled as an RC circuit with the conduction (Ra) and leakage (Rw) resistances and the capacitance of the membrane. The following diagram gives a simplistic treatment of the axon circuit: Consider only the first stage, prior to the second Ra. [7] If there were no current to the capacitor, what would the voltage across the capacitor be in terms of V, Ra and Rw? [Hint: use Ohm’s Law V = IR and take the potential at the lower horizontal trace to be zero.] [8] Is the capacitor charged or discharged when the current stops flowing to it? Applying Kirchoff’s laws to the first stage of the circuit gives the following 3 equations: V- I1R1 - Vc = 0 Vc – I2R2 = 0 I1 = I2 + Ic From the third equation we get Ic = I1 – I2 Solving the first 2 equations for I1 and I2 and substituting the expressions in the previous equation gives (V Vc) Vc Ic R1 R2 1 V 1 Vc Ic R1 R1 R2 The first term is a constant, the second term involves the changing value Vc. The term in brackets is 1/Rll where Rll is the resistance of R1 and R2 in parallel. Multiplying by Rll and writing Ic as ΔQ/Δt = C ΔVc/Δt gives V R R1 Vc R C Vc t Since the RC value in front of ΔVc/Δt is RllC, this is the time constant for the circuit. The first term gives the maximum value for Vc. [9] Write an expression for the potential across the capacitor as a function of time, starting at Vc = 0. [hint, the equation above shows ΔVc/Δt is proportional to Vc.] [10] Extending this treatment, write a similar expression for the second capacitor in line. How about for the nth capacitor? In each step, the V used in the previous question is the Vc from the previous stage. Such behavior agrees semi-quantitatively with data measured on actual neurons. This drop in potential over distance requires amplification of the signal, which is accomplished by triggering the sodium channels to open and replenish the potential strength. Below is a trace of a pulse in the squid axon and some of the characteristics of the axon and signal. Giant Squid Axon, ~ 1mm diameter, unmyelinated. V(signal) ~25m/s (Wik) More discussion can be found on the hyperphysics site: http://hyperphysics.phy-astr.gsu.edu/Hbase/biology/actpot.html Video of original work on Giant Squid Axon: http://www.science.smith.edu/departments/NeuroSci/courses/bio330/squid/squid4%20.html Electric Potential and ion concentration: From the Boltzmann distribution of particles among different energy levels at a given temperature, we have the concentration ratios for inside and outside the cell as q (Vi Vo ) c ci e kT or q(Vo Vi ) kT ln i co co This is another version of the Nernst equation used in Chemistry. This is the source of the potential difference across the axon membrane due to ion concentration differences. An axon in the resting state is much higher in potassium ion concentration than the fluid outside the cell and much lower in both chloride and sodium ions. The additional negative ions inside the axon are amino acid ions. Nerve cells are composed of a cell body, a long axon and projections; dendrites on the cell body and axon terminals on the axon. A few types of neurons are shown at right. Nerve cells communicate using synapses, regions where one cell is a source of neurotransmitter molecules and the other cell has receptors on its surface for these chemicals. When a signal passes down the axon of the sending cell and reaches the bouton at the end of the axon terminal, the cell releases neurotransmitters contained in vacuoles in the cell. These then diffuse across the synaptic gap to the receptors on the surface of the receiver cell and trigger the propogation of the signal through the receiver cell to its axon. Fig. 1 : Neuron types Synapses in cerebral cortex are about 51% axodendritic, 47$ on dendritic spines and 2% axosomatic. [2] In the central nervous system, axoaxonic synapses are common. Rather than connect from the axon terminal boutons to dendrite membranes, axon boutons connect to axon boutons on the reciever cell.Axosomatic synapses where axonic boutons connect to the synapse main body of the receptor neuron occur in the brain. Fig 2 : Chemical Synapse In addition to these chemical synapses, physiologists have found electrical synapses, which are direct connections between cells allowing direct ion flow through pores between two cells. Gaps in such synapses are about 3.5 nm and allow free flow of ions betweeen them without delay. Muscle cells and others, in addition to nerve cells have been found to posess these features.[3] Myelination: Myelinated nerves provide much faster signal transmission than bare nerrve cells. Myelinated cells have Schwann cells wrapped around them at close intervals. Electrically, these function as insulation and there are no ion channels in the neuron walls there. See figure 1, cells on the right. Between the rolled up Schwann cells are the Nodes of Renvier with ion channels. Here, signal strength can be boosted, as we shall see. Signal Propagation A nerve signal, beginning at the bouton on a transmitting cell, starts with the release of neurotransmitter molecules from the transmitting cell. These diffuse across the synaptic gap and bind to receptors on the surface of the receiving cell. This begins internal chemical processes which trigger ion channels in the receiver cell and an electrical signal is generated by shifting charge across the membrane. This electrical signal then propagates down the nerve cell, through the axon to its terminal boutons where the procedure is repeated. We are going to focus on the electrical portion of the process, when the signal is propagating down the nerve cell. Time scale The time scale is set by the rate at which the electric potential across the cell membrane changes. This is shown in figure 3. Fig 3. Activation potential and ion permeability of cell membrane. As the signal propagates down the axon, ion concentraqtions must change in order to give a potential difference. A summary and diagram of the proces is given below. Steps in an Action Potential 1. At rest the outside of the membrane is more positive than the inside. 2. Sodium moves inside the cell causing an action potential, the influx of positive sodium ions makes the inside of the membrane more positive than the outside. 3. Potassium ions flow out of the cell, restoring the resting potential net charges. 4. Sodium ions are pumped out of the cell and potassium ions are pumped into the cell, restoring the original distribution of ions. Fig 4 Potential pulse propagation through axon. (a) Resting state, net positive charge on outside of membrane. (b) Potential pulse has traveled to point P via ion migration as noted by arrows. As the charges at nearby Q diminish, and the potential inside increases to the action potential threshold. (c) Once the threshold is reached, it triggers a protein conformation change allowing Na+ ions to flow into the cell and the potential increases rapidly to a positive value. This moves the pulse of positive potential down the axon. (d) As the pulse passes by, the potential inside the cell again returns to a negative value close to the original resting potential due to an outward flow of K+ ions. The return to the resting state is accomplished over a long period of time by the active Na+ - K+ pump. In myelinated nerve cells, this process is interrupted by the Schwann cells, where there are no ion channels and less drop in charge as the signal propagates. [1] http://www.willamette.edu/~gorr/classes/cs449/brain.html [2] Pappas, George D. and Purpura, Dominick P., Structure and Function of Synapses, New York Society of Electron Microscopists, 1972 , Raven Press, NY. [3] Greenstein, Ben and Adam Greenstein, Color Atlas of Neuroscience: Neuroanatomy and Neurophysiology, 2000, Georg Thieme Verlag, Stuttgart Germany, p.92 Brain, nerves, intelligence http://www.willamette.edu/~gorr/classes/cs449/brain.html http://www2.estrellamountain.edu/faculty/farabee/biobk/BioBookNERV.html Electrical circuit emulations http://physicsandcake.wordpress.com/2010/02/23/josephson-junction-neurons/