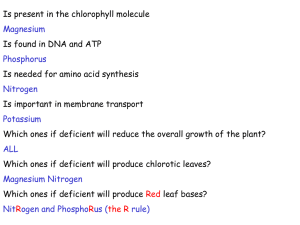
Bar, A., 2009. Calcium transport in strongly calcifying laying birds
advertisement

1 1 Preprint 2 Review 3 Calcium 4 Mechanisms and regulation transport in strongly calcifying laying birds: 5 6 7 Arie Bar1 8 Institute of Animal Science, ARO, the Volcani Ctr., Bet Dagan, Israel 9 10 Running head: Calcium transport in laying birds 11 12 13 14 15 16 17 18 Published in: Comparative Biochemistry and Physiology, Part A: Molecular & Integrative 19 Physiology, (2009) 152: 447-569. 20 For the printed version please link to: 21 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation& 22 list_uids=19118637 23 24 1 Retired; Institute of Animal Science, ARO, the Volcani Ctr., Bet Dagan 50250, Israel; E-mail: ariebar@agri.gov.il 2 25 ABSTRACT 26 Birds that lay long clutches (series of eggs laid sequentially before a “pause day”), among 27 them the high-producing, strongly-calcifying Gallus domesticus (domestic hen) and Coturnix 28 coturnix japonica (Japanese quail), transfer about 10% of their total body calcium daily. They 29 appear, therefore, to be the most efficient calcium-transporters, among vertebrates. Such 30 intensive transport imposes severe demands on ionic calcium (Ca2+) homeostasis, and 31 activates at least two extremely effective mechanisms for Ca2+ transfer from food and bone to 32 the eggshell. 33 This review focuses on the development, action and regulation of the mechanisms 34 associated with paracellular and transcellular Ca2+ transport in the intestine and the eggshell 35 gland (ESG); it also considers some of the proteins (calbindin, Ca2+ATPase, Na+/Ca2+ 36 exchange, epithelial calcium channels (TRPVs), osteopontin and carbonic anhydrase (CA)) 37 associated with this phenomenon. Calbindins are discussed in some detail, as they appear to 38 be a major component of the transcellular transport system, and as only they have been 39 studied extensively in birds. The review aims to gather old and new knowledge, which could 40 form a conceptual basis, albeit not a completely accepted one, for our understanding of the 41 mechanisms associated with this phenomenon. 42 In the intestine, the transcellular pathway appears to compensate for low Ca2+ intake, but 43 in birds fed adequate calcium the major drive for calcium absorption remains the 44 electrochemical potential difference (ECPD) that facilitates paracellular transport. However, 45 the mechanisms involved in Ca2+ transport into the ESG lumen are not yet established. In the 46 ESG, the presence of Ca2+-ATPase and calbindin-two components of the transcellular 47 transport pathway–and the apparently uphill transport of Ca2+ support the idea that Ca2+ is 48 transported via the transcellular pathway. However, the positive (plasma with respect to 49 mucosa) electrical potential difference (EPD) in the ESG, among other findings, indicates that 50 there may be major alternative or complementary paracellular passive transport pathways. 51 The available evidence hints that the flow from the gut to the ESG, which occurs during a 52 relatively short period (11 to 14 h out the 24- to 25.5-h egg cycle), is primarily driven by 53 carbonic anhydrase (CA) activity in the ESG, which results in high HCO3- content that, in 54 turn, “sucks out” Ca2+ from the intestinal lumen via the blood and ESG cells, and deposits it 55 in the shell crystals. The increased CA activity appears to be dependent on energy input, 56 whereas it seems most likely that the Ca2+ movement is secondary, that it utilizes passive 57 paracellular routes that fluctuate in accordance with the appearance of the energy-dependent 58 CA activity, and that the level of Ca2+ movement mimics that of the CA activity. The on-off 59 signals for the overall phenomenon have not yet been identified. They appear to be associated 60 with the circadian cycle of gonadal hormones, coupled with the egg cycle: it is most likely 3 61 that progesterone acts as the “off” signal, and that the “on” signal is provided by the 62 combined effect of an as-yet undefined endocrine factor associated with ovulation and with 63 the mechanical strain that results from “egg white” formation and “plumping”. This strain 64 may initially trigger the formation of the mammillae and the seeding of shell calcium crystals 65 in the isthmus, and thereafter initiate the formation of the shell in the ESG. 66 67 68 Keywords: ATPase, Calbindin, Calcium, Carbonic anhydrase, Eggshell gland, Epithelial 69 calcium channels, Gonadal hormones, Intestine, Pump, Paracellular, Transcellular, Transport, 70 Vitamin D, Uterus 71 4 72 Contents 73 1. Introduction 5 74 2. Proteins involved in calcium transport 10 75 2.1. Epithelial calcium channels (TRPVs) 11 76 2.2. Calbindins 11 77 2.3. Plasma membrane calcium-ATPase (Ca2+-ATPase or PMCA) 20 78 2.4. Na+/Ca2+ exchange 21 79 2.5. Carbonic anhydrase 21 80 2.6. Osteopontin 23 81 82 3. Intestinal calcium absorption in the laying bird 3.1. Overall aspects and methodologies 24 24 83 3.1.1. Methodologies x 84 3.1.2. Site of absorption 24 85 3.1.3. Intestinal absorption of Ca2+ in birds, as influenced by nutrition 86 or physiological status 27 87 3.2. Mechanisms of intestinal absorption 28 88 3.3. Absorption of Ca2+ in the laying bird 31 89 3.3.1. Development of absorption capability 31 90 3.3.2. Circadian calcium absorption 33 91 3.3.3. Bone–the other source of shell calcium 36 92 93 4. Eggshell transport of calcium 37 4.1. Early studies on the evaluation of the capability and regulation of calcium 94 transport in the eggshell gland 37 95 4.2. Eggshell gland cyclic functionality 38 96 4.3. Shell-gland-specific proteins 39 97 4.4. Mechanism of calcium transport in the eggshell gland 40 98 5. Conclusions and speculations 42 99 Acknowledgment 47 References 47 100 101 5 102 1. Introduction 103 A unique characteristic of mammalian reproduction is the ability to maintain a most 104 appropriate and protective environment for embryo development inside the uterus. Other 105 vertebrate species, including the thermo-regulated avian species, lack this evolutionary 106 advantage; at an earlier stage of evolution they developed a semi-protected milieu, the egg, 107 which fulfils most embryo needs, apart from the thermo-regulated environment. The eggshell 108 isolates the internal milieu from external threats, such as dryness or microorganism 109 penetration, and does so quite adequately in nature. However, modern industrialization and 110 environmental contamination exposes avian species to new, "human-made" threats, many of 111 which impair shell integrity, which arouses our renewed interest in the biology of eggshell 112 formation. The fact that human population world-wide consumes about 1012 eggs per year 113 (64.4 million metric tons in 2005 according to the FAO) adds an economic aspect to the 114 subject. 115 Although many domesticated birds lost some of the typical characteristics of wild birds, 116 such as flying, migration capability, or seasonal breeding, they retained other characteristics, 117 such as photosensitivity, circadian rhythms of egg formation, and sequential patterns of 118 laying eggs (clutch). These characteristics, together with the high reproduction rate of the 119 domestic hen (Gallus domesticus) and its economic importance, made this species the 120 commonest model for studying shell formation. Other domesticated, or semi-domesticated, 121 species, such as the duck (Anas platyrhynchos) (Benoit et al. 1944; Lundholm 1991)2, 122 turkey (Meleagris gallopova) (Musser et al. 1977), ostrich (Struthio camelus) (Holm et al. 123 2000), or Japanese quail (Coturnix coturnix japonica), (Bar et al. 1976a; Bar et al. 1976b; 124 Kenny 1976; Musser et al. 1977; Striem et al. 1991; Holm et al. 2001) have also been 125 extensively studied. Among these species the quail appears to be the most similar to the 126 domestic hen with regard to productivity, egg cycle length, shell formation, calcium 127 homeostasis, and cholecalciferol (vitamin D3) metabolism and expression (Tables 1, 2), but 128 not with regard to ovulation time. Because they are small and oviposit and ovulate in the 129 afternoon, and their shell calcification occurs during daylight hours, quail has become an 130 attractive and widely used bird for research on shell calcification, vitamin D metabolism and 131 calcium homeostasis The latter is controlled by three calcium-regulating hormones 132 (calcitropic): parathyroid hormone (PTH; reviewed in (Ingleton 2002; Dack, 2000; Talmage 133 and Mobley, 2008; Potts 2005)), the hormonal form of vitamin D3–the 1,25 dihydroxyvitamin 134 D3 (1,25(OH)2D3; reviewed in (Hurwitz 1992; Norman 1995; Soares et al. 1995; Bouillon et 22 Only few of the relevant reviews or original articles are cited. Efforts have been done to cite in most cases the most recent reviews and the earlier original papers. In some cases are cited other (more recent, more relevant or more comprehensive) publications. 6 135 al. 1997; Henry 1997; Pike and Shevde, 2005; Wasserman 2005; Haussler et al. 1998; Jones 136 et al. 1998; Whitehead 1998; Brown et al. 1999; Edwards 2000; Holick 2003; DeLuca 2004; 137 Dusso et al. 2005; Wasserman 2005; Norman 2006; Christakos et al. 2007; Bar 2008; Khanal 138 et al. 2008, Perez et al. 2008; Perez et al. 2008)) and calcitonin (reviewed in (Silverman 2003; 139 Huang et al. 2006; Ramasamy 2006)). 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 Table 1. Some variables of egg laying in the fowl and quail _____________________________________________________________________________________________ Variable Fowl Quail Reference _____________________________________________________________________________________________ Body weight at production onset, kg Egg cycle length3, h Egg production, eggs per 348 d4 Egg weight, (age of 13-14 m), g Shell Ca2+, g 1.527±0.2301 23 to 28 324±28 67.0±5.3 2.30±0.22 Oviposition, h post lights turned off 5 12 to16 Peak of progesterone, h prior ovulation 6 to 2 0.110 to 2022 24 to 26 309±905 10.3±0.5 0.284±0.031 18 to 26 6 to 2 (Bar et al. 1981b; Bar et al. 1998; Minvielle et al. 2000) (Kobayashi et al. 1981; Etches 1990; Sonoda et al. 1997) (Bar et al. 1998; Minvielle et al. 2000) (Bar et al. 1976b; Bar et al. 1998) (Bar et al. 1976b; Bar et al. 1996) (Kobayashi et al. 1981; Etches 1990; Sonoda et al. 1997) (Furr et al. 1973; Haynes et al. 1973; Lague et al. 1975; Shahabi et al. 1975; Doi et al. 1980; Johnson et al. 1980; Gulati et al. 1981; Kamar et al. 1982; Nys et al. 1986a; Vanmontfort et al. 1994; Braw-Tal et al. 2004) _____________________________________________________________________________________________ 1 Mean ± SD 2 Range for several different breeds 3 Birds maintained under 14L:10D regime (L = light on, D = light off). In 14-month-old fowls maintained at 16L:8D cycle length (±SD) was 24.3 ± 0.6 h (Bar et al., unpublished results) 4 During the age ranges of 153 to 500 and 40 to 397 days, respectively, in breed selected for egg laying. 5 Birds maintained under 14L:10D regime (L = light on, D = light off). In fowls maintained under 16L:8D regime, cycle length (±SD) was 23.6 ± 1.4 h; 73% of the eggs were oviposited within 11 to 15 h following light off (Bar et al., unpublished results) 7 170 171 Table 2. Selected data on the effect of egg laying (a) and shell calcification (b) in the female fowl (Gallus domesticus) and quail (Coturnix coturnix japonica) 1 172 173 A. Effect of egg laying _______________________________________________________________________ 174 175 176 Variable 177 ________________________________________________________________________ 178 179 Plasma calcium , mM 180 181 Plasma 1,25(OH)2D3, nM 182 183 Jejunal (upper villus) VDR4, 184 185 186 187 Duodenal calbindin, mg/g 188 189 Ca2+ absorption5, % of intake 190 191 192 193 ESG6 VDR4, pmol/g 1.40±0.05 2.44±0.20 ESG6 calbindin, mg/g 0.17±0.13 1.14+0.08 194 ________________________________________________________________________ 195 Fowl Immature Quail Laying3 pullet 2.78±0.1 Immature . References2 Laying3 pullet 6.82±0.62 2.7±0.15 6.15±0.32 (Bar et al. 1978b; Singh et al. 1986) 0.11±0.01 0.61±0.08 0.42±0.07 1.38±0.15 (Bar et al. 1981b; Nys et al. 1989) abundance measures 4.3±0.5 0.34±0.09 13.4±1.6 2.16±0.19 (Wu et al. 1993) 0.44±0.16 2.06±0.20 (Bar et al. 1975a; Bar et al. 1976a; Montecuccoli et al. 1977b) 21.3±2.6 50.1±6.23 21.3±2.6 63.9±3.8 (Bar et al. 1978a; Cohen et al. 1978) (Striem 1990) 1.10±0.09 1.70±0.08 (Bar et al. 1975a; Montecuccoli et al. 1977b) 8 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 B. Effect of shell calcification on laying birds fed laying diets (adequate calcium) _______________________________________________________________________ 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 ________________________________________________________________________ Variable Fowl 6 ESG inactivity Quail Shell calcification ESG inactivity References2 . Shell calcification ________________________________________________________________________ Plasma calcium , mM 5.12±0.2 5.89±0.37 6.9±0.2 6.7±0.17 Plasma Ca+2, mM 1.45±0.06 1.31±0.047 0.98±0.088 0.74±0.048 (Kaetzel et al. 1985; Singh et al. 1986) 1.15±0.05 1.04±0.03 (Dacke et al. 1973) Plasma PTH, pg/ml Plasma 1,25(OH)2D3, nM Jejunal VDR4 mRNA, % of 1 h pre ovulation 0.55±0.10 (Singh et al. 1986) 5.95±0.61 0.70±0.07 0.78±0.1 0.53±0.02 0.68±0.05 91 (Bar et al. 1976b; Bar et al. 1996) 0.53±0.12 8 8 0.68± 0.17 (Bar et al. 1984b; Kaetzel et al. 1985) (Nys et al. 1992a) 90 (Ieda et al. 1995) Duodenal calbindin mRNA9, fmol/ng RNA Duodenal calbindin, mg/g 276±12 2.62±0.13 Ca2+ absorption5, % of intake 38.0±8.6 ESG6 VDR4 mRNA, % of 1 h pre ovulation 254±33 2.16±0.19 59.9±5.3 44 400 fmol/ng RNA 48±21 248±.15 ng/g tissue 22±4 514±77 238±43 1.85±0.25 17.8±4.3 404±68 (Striem et al. 1991; Bar et al. 1992a) 2.06±0.20 (Bar et al. 1975a; Bar et al. 1976a; Montecuccoli et al. 1977b) 63.9±3.8 (Bar et al. 1976a; Cohen et al. 1978) (Ieda et al. 1995) ESG6 calbindin mRNA9 ESG calbindin, mg/g 1 1.20±0.08 1.14+0.08 41±10 509±26 (Striem et al. 1991; Bar et al. 1992a) (Nys et al. 1989) 2.88±0.13 2.24±0.15 (Bar et al. 1975a; Bar et al. 1976b; Bar et al. 1978b) The table is intended to bring together quantitative and comparative data. Many other studies, but not all, are mentioned in the text and in the list of references. 2 Respectively. 3 During period of shell calcification. 4 Vitamin D receptor. 5 Intestinal absorption capability of laying hens during shell calcification reaches in some studies much higher (Hurwitz et al. 1973a). 6 Eggshell gland (uterus). 7 Other publications (data given as figures) indicated for a more pronounced differences between calcifying and not calcifying fowls (Nys et al. 1986a; Frost et al. 1990). 8 Calculated from the original graphical presentation. 9 Based on intestinal level at 1 h pre ovulation. 10 Original data were presented as figures. 9 246 Egg laying and shell calcification impose severe extra demands on Ca2+ homeostasis, 247 since shell formation requires several times as much calcium as the amount present in the 248 extra-cellular pool. In the high-laying-rate domestic hen and quail, 2 to 3 g (about 10% of the 249 total body calcium) and approximately 0.3 g, respectively, of Ca2+ is secreted daily during the 250 relatively short period (11-14) h out of the 24-25.5 h cycle) of intensive shell calcification. 251 This complicates the conceptual model of homeostasis that fits non-laying animals (reviewed 252 in (Hurwitz 1990; Miller 1992; Hurwitz 1996; Sasayama 1999; Bar 2008)) by introducing an 253 additional Ca2+ pathway: its withdrawal through the uterus (eggshell gland; ESG). The last is 254 a process that appears not to be controlled by the three calcium-regulating hormones, or at 255 least to be controlled differently from Ca2+ transport in the intestine, bone and kidney 256 (reviewed in (Bar 2008)). 257 The steady state of calcium metabolism in the non-laying bird is challenged, first through 258 the initiation of gonadal activity during maturation prior to egg laying, and then by the 259 increased demands for shell Ca2+ during the egg-laying phase (reviewed in (Dacke 2000; Bar 260 2008)). During maturation the secretion and activity of gonadal hormones create increased 261 Ca2+ requirements, to support new medullary bone (MB) formation and to saturate the 262 estrogen-dependent-plasma-calcium-binding proteins formed in the liver (reviewed in 263 (Griffin 1992; Walzem 1996; Davis 1997; Walzem et al. 1999) and others). 264 The daily Ca2+ intake of the laying hen appears to be 4.2 to 4.6 g/d (based on daily feed 265 intake of approximately 115 g/d and the recommended calcium content of 3.6 to 4.0%). At 266 zero nutritional balance for calcium, and under the theoretical conditions–which are not 267 fulfilled in nature–of constant dietary inflow and shell-deposition outflow of Ca2+, the net rate 268 of dietary Ca2+ absorption in the laying domestic hen should be about 50%. In fact, the actual 269 rate of absorption is greater, because the flow of Ca2+ from the intestine to the plasma occurs 270 mainly when the gut contains calcium derived from the diet, which occurs only during 271 daylight and the early hours of darkness, because eating ceases after sunset or after switching 272 off of the artificial lighting. According to the rate of passage of feed through it (Hurwitz et al. 273 1966b), the intestinal lumen became almost completely empty of calcium 4 to 5 h after the 274 end of the feeding period or after "lights-out". Since intensive shell calcification in the 275 commercial fowl occurs between 9 and 22 h after ovulation, i.e., -1 to 13 h after lights-out; 276 (Table 1), a major proportion of the shell Ca2+ is deposited while the intestine lacks dietary 277 calcium. In order to overcome the lack of synchronization between the circadian availability 278 of dietary calcium and the circadian deposition of calcium into the eggshell, two mechanisms 279 were promoted: (a) enhancement of net absorption of Ca2+ during the early dark period, when 280 feed is still present in the gut; and (b) the activation of an efficient process of bone resorption 281 during the second half of the dark period. Incomplete (Bar et al. 1988; Clunies et al. 1993) 10 282 restoration of the circadian bone loss of Ca2+ in birds laying sequentially long clutches, occurs 283 during the subsequent daylight period, when the intestinal absorption of Ca2+ is enabled by 284 the renewal of calcium intake in the feed. Thus, the source of the daily deposition of shell 285 Ca2+, both direct and indirect (bone mediated) in birds with long clutches, must be the 286 intestine. 287 2. Proteins involved in calcium transport 288 At least four groups of proteins are considered to be involved in calcium transport, namely: 289 calbindins; plasma membrane calcium-ATPases (Ca2+ATPases or PMCAs); Na+/Ca2+ 290 exchangers (NCXs); and epithelial calcium channels (TRPVs). Whereas these four groups of 291 proteins appear to be involved in transcellular transport (see 3.2) of Ca2+, a fifth group, which 292 includes the Tight Junction (TJ) proteins, is believed to be involved in paracellular transport. 293 At least two other groups of proteins are believed to be associated specifically with ESG 294 calcium transport: carbonic anhydrase (CA) and osteopontin (OPN). As some of these 295 proteins appear to be vitamin-D-dependent or -related proteins, their activity is facilitated by 296 another group of proteins, the nuclear vitamin D receptors (nVDR) and the membrane 297 bound/attached VDRs (mVDR). The nVDRs were extensively reviewed (Norman 1995; 298 Bouillon et al. 1997; Henry 1997; Pike and Shevde, 2005; Wasserman 2005; Haussler et al. 299 1998; Jones et al. 1998; Brown et al. 1999; Holick 2003; DeLuca 2004; Dusso et al. 2005; 300 Wasserman 2005; Norman 2006) and were specifically discussed with regard to theirs 301 relevance to the laying bird (Bar 2008). The mVDR and the TJ proteins (reviewed in 302 (Norman et al. 2002; Dusso et al. 2005; Norman 2006) and in (Schneeberger et al. 2004; Van 303 Itallie et al. 2006), respectively) were less studied and were not yet investigated with regard to 304 their relevance to the laying bird. 305 VDRs, TRPVs, and PMCAs were reviewed extensively (see 2.1, 2.3 and (Bar 2008)) and 306 theirs relevance to the laying bird was addressed recently (Bar 2008), therefore they are not 307 addressed or are only briefly discussed in the present review, in which more attention is paid 308 to NCXs and osteopontin, which were not discussed in the earlier review. As CAs appear to 309 be most important for ESG transport of Ca2+, they are discussed widely in the present review, 310 with regard to egg laying also. Special attention is given to avian calbindin as it is the only 311 calcium-transport-related protein that has been studied extensively in birds, because it is 312 considered to be an important component of the transcellular route of Ca2+ transport and 313 because it was used in many studies as a means of estimating Ca2+ absorption. This protein, 314 although widely discussed in an earlier review (Bar 2008) is discussed in the present one with 315 respect to some characteristics that were not addressed in the earlier review, its differential 316 regulation and its possible differential functionalities in the intestine and the ESG. 11 317 2.1. Epithelial calcium channels (TRPVs) 318 The TRP (Transient Receptor Potential) super-family of cation channels is widely 319 distributed in calcium-transporting tissues such as mammalian proximal intestine, kidney and 320 placenta (reviewed in: (Belkacemi et al. 2005; Hoenderop et al. 2005b; Niemeyer 2005; van 321 der Eerden et al. 2005; Lambers et al. 2006a; Venkatachalam et al. 2007 ; Bar 2008)). Briefly, 322 they comprise more than 27 proteins in six subfamilies. TRPV5 and TRPV6 are considered to 323 facilitate the entry of Ca2+ into the epithelial cells of the calcium-transporting organs. Their 324 gene expression in the mammalian intestine and kidney is regulated by 1,25 (OH)2D3 and the 325 genes encoding them have a VDRE (reviewed in (Belkacemi et al. 2005; Brown et al. 2005; 326 Hoenderop et al. 2005b)). Estrogens have a distinct, vitamin D-independent stimulating effect 327 at the genomic level of TRPV6 (reviewed in (Hoenderop et al. 2005a; van Abel et al. 2005)); 328 they also affect renal TRPV5 (Van Cromphaut et al. 2003). The effect of estrogen on TRPV6 329 may be mediated by an estrogen-responsive element (ERE) that has been identified on the 330 promoter sequence of mouse TRPV6, but not on TRPV5 (Weber et al. 2001). The specific 331 role of TRPVs in the transport of Ca2+ in the laying-hen intestine and ESG has not yet been 332 studied. 333 2.2. Calbindins 334 This group of proteins, previously named CaBPs (Ca-binding proteins) was widely 335 reviewed (Gross et al. 1990; Heizmann et al. 1990; Thomasset 1997; Hemmingsen 2000; 336 Christakos et al. 2003; Choi et al. 2005; Christakos et al. 2005). Avian calbindin was recently 337 reviewed with regard to laying status and shell calcification ((Bar 2008) and in Bar, 2009, in 338 preparation). 339 Briefly: High concentrations of calbindins are found in tissues characterized by their 340 massive transport of Ca2+, such as intestine, kidney and avian ESG (Wasserman et al. 1966; 341 Taylor et al. 1967; Corradino et al. 1968). Lower concentrations of calbindins are found in 342 other tissues associated with Ca2+ homeostasis and metabolism; these include bone, tooth 343 cells and PT cells. These proteins are also found in tissues not directly associated with Ca2+ 344 transport: the nervous tissues contain high concentrations; the pancreas and testes contain low 345 concentrations (reviewed in (Christakos et al. 2005). In the calcium-transporting tissues the 346 calbindins are considered to facilitate the movement of Ca2+ within the epithelial cells of the 347 calcium-transporting organs. 348 Recently it was also suggested (reviewed in (Lambers et al. 2006b; Christakos et al. 349 2007)) that calbindins may act as a buffer, by maintaining a low Ca2+ concentration in close 350 proximity to the TRPVs pores, and thereby ensuring the "downhill" movement of Ca2+ into 351 the cell. Although these proteins appear to be primarily associated with Ca2+ transport, they 12 352 may also be involved in protecting the cells from high concentrations of Ca2+ or from 353 apoptotic cellular degradation (Christakos et al. 2003). 354 Unlike the mammalian intestine, uterus and placenta, which contain mainly calbindin D9K, 355 the avian tissues contain almost only calbindin D28K. In the chick intestine and ESG, the 356 protein is localized primarily in the absorptive cells and in the tubular gland cells, 357 respectively (Lippiello et al. 1975; Jande et al. 1981; Wasserman et al. 1991). Renal calbindin 358 D28K is exclusively localized in the distal convoluted and collecting tubules (reviewed by 359 (Raval-Pandya et al. 1999; Christakos et al. 2005). In the chicken and quail intestine, 360 calbindin concentrations are higher in the proximal than in the distal segments (Taylor et al. 361 1967; Bar et al. 1976b). In all three major-Ca2+-transporting organs, calbindin content is 362 closely correlated with Ca2+ transport (Taylor et al. 1969; Morrissey et al. 1971; Bar et al. 363 1975a; Bar et al. 1979a; Bar et al. 1984b). However, whereas the calbindin contents in the 364 kidney and in the ESG are correlated with the mass of calcium transported (weight unit), the 365 intestinal calbindin is correlated with calcium transport capability (percentage absorption, 366 Fig. 1) rather than with the mass of calcium absorbed (Fig. 2) (Bar et al. 1979a; Bar et al. 367 1979b; Bar et al. 1984b; Bar et al. 1992b)). 368 Avian intestinal, ESG and kidney calbindins are identical (Taylor et al. 1972; Bar et al. 369 1976a; Fullmer et al. 1976), comprise 261 amino acids (Wilson et al. 1985; Hunziker 1986; 370 Fullmer et al. 1987) and contain six EF hands, four of which bind Ca2+ strongly. The apparent 371 Ka is 108M-1 to 106M-1. Lower affinity was observed for other cations, diminishing in the 372 order Ca2+> Cd2+> Sr2+> Mn2+> Zn2+> Ba2+> Co2+> Mg2+ (Ingersoll et al. 1971). The 373 calbindin D28K gene is about 19 kb long, consists of 11 exons (Minghetti et al. 1988; Wilson 374 et al. 1988) and is highly conserved in evolution. A putative vitamin D-responsive element 375 (VDRE) was identified on the mouse calbindin D28K promoter. Some observations also 376 indicate the presence of putative low active or inactive VDRE on the chicken gene encoding 377 calbindin D28K (reviewed in (Christakos et al. 1997; DeLuca 2004; Christakos et al. 2005)). In 378 addition an estrogen-responsive element was also identified on the 5'-flanking region of the 379 calbindin D28K promoter (Gill et al. 1995; Criddle et al. 1997). 380 381 13 382 383 384 385 386 387 388 389 Fig. 1. (upper panel) Relationship between duodenal calbindin and intestinal capability to absorb calcium in non-laying and laying hens during periods of shell calcification (from (Bar et al. 1979a)). CaBP, calbindin, Ca, total calcium); and (lower panel) Relationship between eggshell gland (EGS) calbindin and shell calcium of laying hens during periods of shell calcification (with permission from (Bar et al. 1984b)). 14 390 391 392 Fig. 2. Plasma calcium, intestinal and plasma calbindin, and intestinal calcium absorption as functions of calcium intake (with permission from (Bar et al. 1979b)). 393 394 At least in the avian intestine, kidney, ESG and brain, three species of mRNAs are 395 encoded. A major one (approximately 2.0 kb) and two minor species (approximately 2.7 and 396 3.0 kb, (Fig. 3) all encode calbindin D28K. However, despite their similarity, the synthesis of 397 avian calbindin is regulated differently (Table 3) in each of the three major transporting 398 organs (for details and references see (Bar 2008) and Bar, 2009, submitted). 399 Intestinal calbindin mRNAs and calbindin synthesis reflect the changes in 1,25(OH)2D3 in 400 the intestinal cell, whether these changes result from exogenous supplementation of vitamin 401 D derivatives (Wasserman et al. 1966; Bar et al. 1975b; Bar et al. 1976b; Bar et al. 1978b; 402 Bar et al. 1990b; Striem et al. 1991; Bar et al. 1992a) or occur in response to restrictions of 403 dietary Ca2+ and/or P (Wasserman et al. 1968; Morrissey et al. 1971; Bar et al. 1972b; Bar et 404 al. 1973a; Friedlander et al. 1977; Montecuccoli et al. 1977a; Bar et al. 1984a; Bar et al. 405 1990a), growth, age (Bar et al. 1981a; Bar et al. 1988; Bar et al. 1999; Bar et al. 2003), 406 maturation, or onset or arrest of laying (Wasserman et al. 1968; Bar et al. 1972a; Striem et al. 407 1991; Bar et al. 1992a; Nys et al. 1992a; Sugiyama et al. 2007). 15 408 409 410 411 412 413 414 415 416 417 418 419 420 421 422 423 Fig. 3. Visualization of calbindin mRNAs from the duodenum and eggshell gland (ESG) by Northern blotting. (a) Effect of laying on duodenal calbindin mRNAs: Lane 1, vitamin-D-deficient chick; lane 2, mature non-laying; lane 3, laying hen (from (Bar et al. 1992a)). (b) Effect of laying on ESG calbindin mRNAs: Lane 1, laying hen duodenum; lane 2, ESG of mature non-laying; lane 3, ESG of laying hen during period of ESG inactivity; lane 4, ESG of laying hen during period of shell calcification (from (Bar et al. 1992a)). (c) Effect of shell calcification blocking of ESG calbindin mRNAs: Lane 1, untreated laying hen; lane 2, laying hen treated with a single oral dose of the carbonic anhydrase inhibitor acetazolamide; lane 3, laying hen following forced immature oviposition (from (Bar et al. 1998)). (d) Effect of shell quality: Lane 2, laying hen with shell-less eggs; lane 3, laying hen with normal eggs; lane 4, laying hen with broken or cracked eggs; above are shown the previous last eggs, Hens were sampled 17 h post oviposition (from (Bar et al. 1998). Membranes were hybridized with oligonucleotide complementary to the mRNA sequence encoding amino acids 58-68 of chicken calbindin (with permission). Similar results were obtained using the quantitative hybridization assay". “solution 16 424 425 426 427 428 429 430 431 432 433 434 435 436 437 438 439 Table 3. Differential regulation of avian calbindin: Effects of selected physiological and nutritional alterations ___________________________________________________________________________ 440 441 442 443 444 445 446 447 448 449 450 451 452 453 454 455 456 457 458 459 460 461 462 463 464 465 466 467 468 469 470 471 472 Cause/tissue Intestine Kidney ESG References1 ___________________________________________________________________________ Vitamin D derivatives in vitamin-D-deficient2 +++3 ++ =/+4 (Wasserman et al. 1966; Corradino et al. 1968; Taylor et al. 1972; Striem et al. 1991) 1-hydroxylated derivatives in vitamin-D-fed +++ = = (Bar et al. 1973c; Bar et al. 1975b; Bar et al. 1976b) Dietary Ca2+ restriction in vitamin-D-fed +++ =/- =/- (Wasserman et al. 1968; Bar et al. 1972a; Bar et al. 1973b; Bar et al. 1975b; Bar et al. 1978b) Dietary P restriction in vitamin-D-fed +++ +++ = (Morrissey et al. 1971; Bar et al. 1973c; Bar et al. 1975b; Bar et al. 1984a) = = ND5 (Bar et al. 1973c; Bar et al. 1975b) +++ +++ ND (Bar et al. 1973c; Bar et al. 1975b) - + ND (Bar et al. 1990a) Growth ++ = ND (Bar et al. 1981a) Maturation or gonadal hormones ++ = =/+ (Bar et al. 1972a; Bar et al. 1973b; Montecuccoli et al. 1977b; Bar et al. 1978b; Bar et al. 1979a; Navickis et al. 1979a; Navickis et al. 1979b) Laying +++ = +++ (Corradino et al. 1968; Wasserman et al. 1968; Bar et al. 1972a; Bar et al. 1973b; Bar et al. 1978b) Shell calcification (on protein synthesis) = ND = Shell calcification (on mRNA synthesis) = ND +++ Dietary Ca2+ restriction in 1-hydroxylated-D-fed Dietary P restriction in 1-hydroxylated-D-fed High dietary Ca2+ in D-fed (Bar et al. 1975a) (Nys et al. 1989; Striem et al. 1991; Bar et al. 1992a; Nys et al. 1992a) __________________________________________________________________________ 1 The table tries to bring together most earlier published quantitative or semi-quantitative comparisons, as well as supporting evidence published later by other research teams. Many, but not all, other studies are mentioned in the text and in the References list. Few of the very early studies used the chelex assay, but were confirmed later with immunoassays or Western analysis. 2 Not laying. 3 The regulative response varied between non (=) to very strong (+++) or negative (-); ND, not detected; 4 Varied in accordance with laying rate. 5 Not determined. 17 473 As in the intestine, the full modulation of renal calbindin mRNAs requires vitamin D. 474 However, unlike intestinal calbindin, renal calbindin did not completely disappear in vitamin 475 D-deficient birds, and even retained part of its capability to be modulated in response to 476 dietary alteration (Bar et al. 1975b; Bar et al. 1990a). Restriction of dietary P, but not of 477 dietary calcium, as well as high dietary calcium, induced also the synthesis of renal calbindin 478 D28K but not of ESG calbindin D28K in birds (Bar et al. 1975b; Bar et al. 1984a; Rosenberg et 479 al. 1986; Bar et al. 1990a). Whereas none of the observed changes in intestinal calbindin and 480 its mRNAs could be attributed to changes in plasma Ca2+ content, renal calbindin mRNA and 481 calbindin were positively related to plasma Ca2+ (Taylor et al. 1972; Rosenberg et al. 1986; 482 Bar et al. 1990a) and to urinary Ca2+ excretion, independently of vitamin D status (Bar et al. 483 1975b; Rosenberg et al. 1986; Clemens et al. 1989; Bar et al. 1990a). The above findings do 484 not support the hypothesis that renal calbindin is involved in Ca2+ reabsorption, especially in 485 light of the fact that it is induced when the body is loaded with calcium (in P-restricted birds 486 or in those fed high-calcium diets). 487 Most of the available evidence does not support the idea that ESG calbindin D28k is 488 vitamin D dependent: endogenous or exogenous 1,25(OH)2D3 (Bar et al. 1976b; Bar et al. 489 1988; Bar et al. 1990b), as well as dietary alterations (Bar et al. 1973b; Bar et al. 1978b; Bar 490 et al. 1984a; Bar et al. 1984b; Bar et al. 1999; Ieda et al. 1999), had no effect on ESG 491 calbindin, whereas they did affect intestinal or renal calbindin; furthermore, ESG calbindin 492 mRNAs are induced in the shell-forming, vitamin D-deficient quail (Striem et al. 1991). 493 In the female bird, intestinal calbindin mRNA (Striem et al. 1991; Bar et al. 1992a) and 494 calbindin (Bar et al. 1972a; Montecuccoli et al. 1977b; Bar et al. 1978a; Bar et al. 1981b; Bar 495 et al. 1992a; Nys et al. 1992a; Wu et al. 1994; Bar et al. 1996) are moderately increased 496 during sexual maturation. Treatment with estrogen and testosterone in combination may 497 mimic this effect (Bar et al. 1979a; Navickis et al. 1979a; Nys et al. 1984c). However, the 498 ESG remains in a refractory state prior to actual reproduction, and calbindin mRNAs and 499 calbindin begin to appear during calcification of the first eggshell (Bar et al. 1973b; Bar et al. 500 1978a; Bar et al. 1978b; Striem et al. 1991; Bar et al. 1992a). In the ESG, but not the 501 intestine, calbindin mRNAs oscillate during the diurnal egg cycle, between near-zero and 502 high concentrations, in close temporal association with eggshell calcification (Figs.3, 4, 5) 503 (Nys et al. 1989; Striem et al. 1991; Bar et al. 1992a; Bar et al. 1992b; Nys et al. 1992b; Ieda 504 et al. 1995)). 505 18 506 507 508 509 510 Fig. 4. The circadian changes in duodenal and eggshell gland calbindin-mRNA and calbindin. , calbindin; , mRNA; , shell calcium (Ca). Mean ± SE. Means designated by different letter are significantly different (P < 0.05) (with permission from (Bar et al. 1992a)). 19 511 512 a c b 513 514 515 516 517 518 d 519 520 521 522 523 524 525 526 527 528 529 530 531 532 533 534 535 Fig 5. The formation and degradation of calbindin and calbindin mRNA in the duodenum and the eggshell gland (ESG) of the laying hen. (a) Degradation during an induced arrest of egg production or (b) log of calbindin concentrations during an induced arrest of egg production. , duodenum; , ESG. (c) Onset of egg formation. Triangulars, calbindin; circles, mRNA; (closed or open symbols are trials 1 & 2, respectively). (d) Model simulation of the synthesis of ESG calbindin mRNA and calbindin at the onset of production. A simulation algorithm was used; the estimated parameters adequately account for the apparent lack of an observed variation in ESG calbindin during the laying cycle in spite of the wide oscillations in the mRNA, as well as the changes in calbindin at the onset of laying. The half-times of calbindin mRNA in the duodenum and shell gland were estimated at 2 and 3.6 h, respectively, and those of calbindin at 13.9 and 32.6 h, respectively. The formation rates of calbindin mRNA were 0.37 and 0.17 pmol.h-1.g-1 and the rate of calbindin formation was 0.099 and 0.031 g.pmol.mRNA-1.h-1 in the duodenum and shell gland, respectively. (with permission from (Bar et al. 1992a)). 536 537 Onset of laying markedly increased intestinal and ESG calbindin mRNA (Nys et al. 1989; 538 Bar et al. 1990b; Striem et al. 1991; Bar et al. 1992a; Nys et al. 1992a) and calbindin 539 (Wasserman et al. 1968; Bar et al. 1973b; Bar et al. 1976a; Bar et al. 1978a; Bar et al. 1978b; 540 Bar et al. 1992a; Sugiyama et al. 2007) synthesis in the intestine and the ESG. Early onset of 541 production is associated with a higher duodenal calbindin concentration than in late layers, 542 most likely as a result of a more severe physiological Ca+2 deficiency (Bar et al. 1972a; Bar et 543 al. 1973b; Bar et al. 1998). 544 Molting (Heryanto et al. 1997; Yosefi et al. 2003) and any other factor that arrests egg 545 production (Bar et al. 1973b; Bar et al. 1973a; Bar et al. 1992a) markedly reduce the intestinal 20 546 and ESG calbindin contents. Following molt, intestinal calbindin content was similar to or 547 even slightly lower than that prior to molt induction (Yosefi et al. 2003). 548 Similarly to duodenal-calbindin, ESG-calbindin is lower in non-laying hens or in hens 549 laying shell-less eggs than in those laying calcified eggs, and is lower in hens that form thin 550 eggshells than in those that form thick ones (Bar et al. 1984b; Nys et al. 1986b; Bar et al. 551 1988; Rabon et al. 1991; Bar et al. 1992a; Bar et al. 1992b; Kang et al. 1996; Bar et al. 1999; 552 Goto et al. 2002c). 553 In most studies estrogens (Navickis et al. 1979b; Nys et al. 1989; Striem et al. 1989; Bar 554 et al. 1990b; Striem 1990; Nys et al. 1992b; Corradino 1993; Corradino et al. 1993; Bar et al. 555 1996) only slightly induced calbindin D28K synthesis in the avian ESG (one to two orders of 556 magnitude smaller than that induced by shell calcification). Progesterone specifically 557 inhibited ESG calbindin synthesis (Bar et al. 1996; Goto et al. 2002b), an effect that was 558 markedly stronger than the observed effects of estrogens, which indicates that it was not due 559 its anti-estrogenic nature. 560 The accumulated evidences suggests that Ca2+ transport in the ESG, similarly to that in 561 the kidney, plays a major role in the synthesis of ESG calbindin mRNAs. It was hypothesized 562 that the regulative mechanism for the synthesis of calbindin mRNAs in the ESG is a complex, 563 multiple one, which involves, in addition to a major Ca2+-transport-related process, estrogen 564 and other endocrine factors (Nys et al. 1989; Striem et al. 1991; Bar et al. 1992a; Nys et al. 565 1992b; Corradino 1993; Corradino et al. 1993; Corradino et al. 1995; Bar et al. 1996; Bar et 566 al. 1999). 567 None of the reproductive factors–including maturation and onset of laying–that modulate 568 intestinal or ESG calbindin was found to affect renal calbindin synthesis in birds (Bar et al. 569 1978b). 570 2.3. Plasma membrane calcium-ATPase (Ca2+-ATPase or PMCA) 571 The PMCAs are a group of more than 30 isomers that use the energy stored in ATP to 572 extrude Ca2+ out of the cell against the electrochemical gradient (reviewed in (Davis et al. 573 1987; Wasserman et al. 1992; Bouillon et al. 2003; Stokes et al. 2003; Belkacemi et al. 2005; 574 Hoenderop et al. 2005b; Nijenhuis et al. 2005)). Briefly, the PMCA1b is the predominant 575 isomer expressed in the mammalian intestine, kidney and placenta (reviewed in (Howard et 576 al. 1993; Nijenhuis et al. 2005)) and the chicken intestine (Melancon et al. 1970; Strittmatter 577 1972; Davis et al. 1987) and kidney (Qin et al. 1993). In the intestine, kidney and placenta the 578 PMCAs are located on the basolateral membrane of the epithelial cell toward which Ca2+ is 579 transported (Borke et al. 1989a; Borke et al. 1989b; Borke et al. 1990). 21 580 The intestinal expression at the transcriptional level is modulated by vitamin D (reviewed 581 in (Zelinski et al. 1991; Wasserman et al. 1992) and also by a variety of factors that affect 582 vitamin D metabolism (Wasserman et al. 1992; Cai et al. 1993; Armbrecht et al. 1994; Zhu et 583 al. 1998). In addition a VDRE sequence was identified in the PMCA1 gene (Glendenning et 584 al. 2000). A few evidence support also the idea that estrogens also regulate PMCAs: (Dick et 585 al. 2003; Van Cromphaut et al. 2003; Van-Abel et al. 2003). 586 In laying birds PMCA(s) are found in the intestine and in the ESG. In the ESG they are 587 localized primarily in the apical-microvillar membrane facing the ESG lumen, rather than in 588 the basolateral membrane. The association of PMCA with Ca2+ transport, its dependency on 589 1,25-(OH)2D3 and estrogens were addressed previously in a recently published review (Bar 590 2008). 591 2.4. Sodium-calcium (Na+/Ca2+) exchange 592 The Na+/Ca2+ exchange mechanism (NCX) is a second transporting system involved in the 593 "uphill" extrusion of Ca+2 across the basolateral membrane of the epithelial cell, toward the 594 extracellular pool. At least three genes encode these transporter proteins in wide a variety of 595 mammalian cells (reviewed in (Belkacemi et al. 2005; Hoenderop et al. 2005b; Lambers et al. 596 2006a; Lytton 2007; van de Graaf et al. 2007; Perez et al. 2008)), but only NCX1 is widely 597 expressed in the calcium-transporting organs, i.e., the intestine, kidney and placenta. In 598 mammalian enterocytes, NCXs do not appear to play a major role in Ca2+ extrusion, whereas 599 the PMCA appear to be the main mechanism by which Ca2+ is extruded from the cells at the 600 basolateral surface. On the other hand, the kidney basolateral Ca2+ efflux is mainly mediated 601 by NCX1, and NCX1 appears to be regulated by Ca2+ (reviewed in (Lytton 2007)). The 602 calcium-regulating hormones, 1,25(OH)2D3 and PTH were found to regulate renal NCX1 603 mRNA synthesis and NCX1 activity, respectively. On the other hand, in an earlier study 604 (Ghijsen et al. 1983) rat intestinal NCX activity was not found to be affected by 1,25(OH)2D3. 605 NCXs were found also in the avian osteoblasts, in the chick embryo heart (Stains et al. 606 2002; Shepherd et al. 2007), and in the chick intestine, where it was induced in response to 607 calcium deficiency (Centeno et al. 2004). They have not yet been determined in the other 608 avian calcium-transporting organ, the ESG. 609 2.5. Carbonic anhydrase 610 The carbonic anhydrases (CAs) are a group of zinc-containing enzymes that catalyze the 611 reversible hydration of carbon dioxide. The CAs are involved in bone resorption and 612 calcification, ion transport, acid-base metabolism, and the movement of respiratory gases. 613 The CA family comprises three evolutionarily unrelated subfamilies without significant 614 sequence homology. At least 16 different isomers were identified in mammalian tissues 22 615 (reviewed in (Esbaugh et al. 2006; Purkerson et al. 2007)) and several novel isozymes have 616 also been identified in avian tissues (Holmes 1977). The commonest of these isomers in avian 617 tissues is CA-II (Holmes 1977). CAs were found in the avian kidney (Holmes 1977; Brown et 618 al. 1982; Gabrielli et al. 1998), epiphysis (Dulce et al. 1960)– specifically in bone osteoclasts 619 (Gay et al. 1974; Billecocq et al. 1990)– intestine (Nys et al. 1984b; Grunder et al. 1990; 620 Gabriella et al. 1994) and ESG (Benesch et al. 1944; Bernstein et al. 1968)– specifically in 621 the ESG epithelial cells (Arai et al. 1996). The CAs are present also in other cells, such as 622 gastric parietal cells and salivary glands, where their main role is to generate H+ and HCO3- 623 during acid-base regulation. The formation of HCO3- in the avian ESG appears to be of 624 especial importance for the deposition of CaCO3 in the shell, where it acts as the sole counter 625 ion for Ca2+. The dependency of CA on vitamin D is not yet clear: a VDRE region was 626 identified in the CA-II gene (Quelo et al. 1994), and 1,25(OH)2D3 was found to regulate the 627 transcription of CA-II in myelomonocytes (Lomri et al. 1992), and to induce their 628 differentiation ((Billecocq et al. 1990; Lomri et al. 1992). Vitamin D3 deficiency caused a 629 reversible reduction in CA activity in the ESG of the laying hen (Grunder et al. 1990), but CA 630 activity was found to be unrelated to plasma level, or to exogenous supplementation of 631 1,25(OH)2D3 (Nys et al. 1986b; Grunder et al. 1990). 632 Maturation and laying were associated with enhanced ESG CA-II activity (Pearson et al. 633 1977; Nys et al. 1986b; Kang et al. 1996), an association attributed mostly to estrogen 634 priming of the ESG (Holm et al. 2001). Molting inhibited CA activity in the ESG (Pearson et 635 al. 1977). Some (Ohashi et al. 1984; Kang et al. 1996), but not all (Grunder et al. 1976; 636 Salevsky et al. 1980; Balnave et al. 1992) publications have suggested that ESG-CA is higher 637 in hens that form thick eggshells than in those that form thin ones, but many of the factors 638 that cause shell thinning in wild birds are associated with reductions in ESG CA-II activity 639 (Lundholm 1997; Holm et al. 2006). The pattern of changes in ESG CA activity during the 640 egg cycle is also a subject of controversy (Salevsky et al. 1980; Kansal et al. 1984; Nys et al. 641 1986b; Balnave et al. 1992). Other factors known to affect ESG CA are the ambient 642 temperature and salinity in the drinking water (Kansal et al. 1984; Yoselewitz et al. 1989; Gill 643 et al. 1990; Balnave 1993; Khalafalla et al. 1998). 644 CA inhibitors, such as acetazolamide, rapidly (10 to 12 h after a single administration in 645 vivo) blocked shell calcification (Bernstein et al. 1968; Pearson et al. 1977; Eastin and 646 Spaziani. 1978b; Bar et al. 1992a; Bar et al. 1999) in spite of the availability of dietary 647 calcium and vitamin D3; they also reduced ESG and intestinal calbindin (Bar et al. 1992a; Bar 648 et al. 1999) and ESG Ca2+-ATPase (Lundholm 1990). Of interest is the finding that 649 acetazolamide in vitro reduced CA activity, but not Ca2+ transport (Ehrenspeck et al. 1971). 650 This is indicative of a possible indirect role of CA in Ca2+ transport. Thinning of the quail 23 651 eggshell caused by p-p'-DDT and DDE was associated with a reduction in ESG AC activity 652 (Bitman et al. 1970). 653 Lower concentrations of CA were found also in the intestinal mucosa of birds (Holmes 654 1977; Gabriella et al. 1994; Elbrond et al. 2004), but whereas the ESG CA activity was found 655 to be affected by the suppression of eggshell calcification, the intestinal activity remained 656 unchanged (Nys et al. 1984a; Nys et al. 1984b). The current models of intestinal Ca2+ 657 transport (see 3.2) do not consider the involvement of CA, therefore, in more recent studies 658 only the changes in ESG CA, but not those in intestinal CA were determined. ((Grunder et al. 659 1990) and many others). 660 2.6. Osteopontin 661 Osteopontin (OPN) is a glycosylated, highly phosphorylated protein (reviewed in (Sodek 662 et al. 2000) and others), expressed in a variety of tissues that are characterized by Ca2+ 663 transport, such as the bone (reviewed in (Butler 1989; Butler et al. 1996; Nakamura et al. 664 2003)), kidney (Rittling et al. 1999) and the ESG (Pines et al. 1995), and also in other cells 665 and tissues, including the immune system and tumors. OPN appears to have roles in wound 666 healing, inflammatory responses and bone remodeling, and it stimulates cellular signaling via 667 various receptors in many cells; these include bone cells, in which it appears to affect 668 migration and maturation of osteoclast precursors, attachment of osteoclasts to the mineral 669 phase of the bone, and osteoclast activity. OPN appears to be one of the major phosphorylated 670 proteins of the avian eggshell matrix (Mann et al. 2007); it is synthesized in and secreted from 671 the isthmus and the ESG of the laying bird (Pines et al. 1995; Lavelin et al. 1998; Lavelin et 672 al. 2000; Fernandez et al. 2003). Expression of ESG OPN, similarly to that of calbindin and 673 other ESG proteins, exhibited diurnal changes, and peaked during shell calcification (Pines et 674 al. 1995). However, whereas calbindin gene expression was stimulated by Ca2+ transport, 675 OPN expression was stimulated by the mechanical strain imposed by the forming egg 676 (Lavelin et al. 1998). No OPN gene expression was detected in the ESG of a pre-laying hen, 677 before the onset of reproduction, or after forced removal of the egg just before its entry into 678 the ESG. OPN was found to be synthesized by the epithelial cells of the ESG that line the 679 lumen. The synthesized OPN is immediately secreted out of cells and accumulates in the 680 eggshell. The presence of OPN in the organic matrix of the shell, especially in the mammillea 681 (mamillary cores (Fernandez et al. 2003; Chien et al. 2008b), the localization of the gene 682 encoding its formation specifically in the ESG cells, its circadian expression in the ESG, and 683 its occurrence in oviduct regions, coincided with the concomitant presence of the egg in each 684 region, and its involvement in calcification of the bone suggests that OPN may play a role in 685 shell calcification (Lavelin et al. 2000). 24 686 The definitive role of OPN in shell formation has not yet been established; expression of 687 its gene is stimulated by many growth factors and hormones, including 1,25(OH)2D3 (Prince 688 et al. 1987; Han et al. 2003). In addition, a DNA sequence responsible for binding VDR-RXR 689 heterodimer was identified on the OPN gene (Noda et al. 1990). However, and as noted above 690 with regard to calbindin, the presence of a VDRE on the gene promoter of CA does not 691 necessarily mean that the gene is induced in the ESG by 1,25(OH) 2D3. Although the 692 mechanism of shell biomineralization is poorly understood, the involvement of OPN in this 693 mechanism may be mediated via the fabrication of OPN-containing fibers (Fernandez et al. 694 2004; Chien et al. 2008,2009) in the collagen type X net of the outer shell membrane that is 695 formed in the isthmus. The mammilea, aggregates of organic material in which shell 696 crystallization is initiated, are then deposited in the isthmus. They consist of an OPN base and 697 a surface containing a calcium-binding keratan sulfate (mammillan). Ovoglycan (dermatan 698 sulfate, another matrix protein) may be also involved as modulator of the crystal growth. The 699 latter two proteins may be just as important as OPN in shell formation; however, the temporal 700 regulation of OPN hints at its possible involvement in the "on" signal for shell formation, as a 701 result of the mechanical strains imposed by egg-white formation in the magnum or by 702 "plumping" in the ESG. 703 3. Intestinal calcium absorption in the laying bird 704 3.1. Overall aspects and methodologies 705 3.1.1. Methodologies: Evaluation of intestinal Ca2+ absorption addresses either the capability 706 of the intestine to absorb Ca2+ (rate of absorption expressed as percentage of dietary or 707 medium content) or the mass of net or true absorption. Table 4 presents a few published 708 observations obtained in laying birds in vivo. These and other observations (Fig. 2; (Bar et al. 709 1979b)) indicate that the rate of absorption does not necessarily correspond to the actual mass 710 absorbed, as the latter depends on the dietary contents. 711 25 712 713 714 715 716 717 718 719 720 721 722 723 724 725 726 727 728 729 730 731 732 733 734 735 736 737 738 739 740 741 742 743 744 745 Table 4. Some factors affecting intestinal Ca2+ absorption in vivo1 in the laying fowl (Gallus domesticus) and quail (Coturnix coturnix) __________________________________________________________________________________________ Factor Fowl Quail References2 2 3 Rate Mass Rate Mass __________________________________________________________________________________________ 1-hydroxylated D3 metabolites in D fed birds3 + + + ND4 (Bar et al. 1976b; Bar et al. 1978b) Low dietary Ca2+ 3 + - ND ND (Hurwitz et al. 1965; Hurwitz et al. 1966a; Bar et al. 1984a) Low dietary P + + ND ND (Bar et al. 1984a) + ND ND ND (Nys et al. 1984c) + + + ND (Hurwitz et al. 1965; Hurwitz et al. 1967a; Hurwitz et al. 1973a; Bar et al. 1978a) + ND ND ND (Nys et al. 1980), + + + ND (Hurwitz et al. 1965; Bar et al. 1976b) = ND = ND (Wasserman et al. 1978; Nys et al. 1980) =/+ + ND ND Gonadal hormones in immature female chicken (in situ) 5 Laying Laying (in situ) 5 Shell calcification (in vivo) 6 Shell calcification (in situ)5 Shell less (or thin shells) eggs3 (Hurwitz et al. 1967a; Waddington et al. 1989) __________________________________________________________________________________________ 1 Measured either with the balance technique or with the non-absorbed reference substances (NARS). 2 Most earlier. 3 These finding were confirmed in situ (Nys et al. 1984c). 4 Not detected. 5 Measured with either tied or perfused intestinal loops. 6 This in vivo finding was confirmed by other groups of researchers who used either the NARS or other methodologies (see 3.11 and 3.3.2 in text) 746 Earlier studies in mammals used a variety of methods, including: in vitro everted gut sacs 747 (Schachter et al. 1959; Harrison et al. 1960) or perfused loops, in situ-tied intestinal sacs 748 (Wasserman 1962; Wasserman et al. 1968; Wasserman et al. 1978) or Thiry Vella 749 (Nicolaysen 1943; Cramer et al. 1962) procedures. These procedures enabled evaluation of 750 the capability of the various intestinal segments to absorb Ca2+, or of the role of vitamin D in 751 this process, or elucidation of the mechanisms involved in Ca2+ absorption, rather than 752 estimation of net or true absorption. Determination of the latter requires the employment of 753 either the in vivo balance technique (Lengemann et al. 1957) or the use of non-absorbed 754 reference substances (NARS) (Marcus et al. 1962). 755 The few in vitro studies of birds yielded only little information. They addressed the 756 intestinal uptake (Sallis et al. 1962; Hurwitz et al. 1967b; Bar et al. 1969b; Bar et al. 1969a; 757 Holdsworth et al. 1975; Al Batshan et al. 1994; Wu et al. 1994) rather than the transfer of 758 Ca2+, because the chicken gut wall in vitro did not permit complete transfer of Ca2+ from the 26 759 mucosal to the serosal medium. Only ileal sacs of young chicks, which had been incubated in 760 a medium low in Na+ (Hurwitz et al. 1967b), exhibited mucosal to serosal transport (as 761 indicated by a serosal/mucosal ratio greater than 1) and a considerable effect of vitamin D. 762 However, in situ observations performed in the same study ((Hurwitz et al. 1967b) and in 763 others (see 3.1.2) identified the upper intestine as the main site of Ca2+ absorption in birds. 764 Much more information was obtained by means of in vitro everted upper (duodenum and 765 jejunum) intestinal sacs prepared from the chick embryo (Corradino 1973; Corradino et al. 766 1991). These in vitro studies confirmed the in vivo identification of the upper intestine as the 767 major absorption site, and thereby enabled focusing of subsequent studies on that region. In 768 addition, these studies provided valuable information on the vitamin D dependency of the 769 absorption process and on its regulation in birds, but not on its dependency on the nutritional 770 or physiological status. 771 In order to gain physiological information in situ-tied or -perfused intestinal loops were 772 used (Wasserman 1962; Hurwitz et al. 1967b; Morrissey et al. 1971; Bar et al. 1974; Nys et 773 al. 1980; Radwan et al. 1985; Annaka et al. 1989), alone or in combination with microscopic 774 imaging (Chandra et al. 1990; Fullmer et al. 1996). These in situ studies addressed the 775 vitamin D dependency of intestinal calcium absorption mechanisms, the site of absorption 776 and the effects of restrictions of dietary Ca2+ or P. The results obtained in these studies 777 enabled the in vitro findings to be extrapolated to the whole bird, but still addressed only the 778 capability to absorb Ca2+ rather the effects of various nutritional or physiological factors on 779 the actual absorption in vivo. Such factors include alteration of dietary composition and the 780 laying status. 781 In order to gain information on the true or net absorption (either capability or actual) as 782 well as on the effects of various nutritional or physiological factors, several in vivo 783 methodologies have been applied. Some studies employed constant feeding (Driggers et al. 784 1949) or single intubations (Clunies et al. 1993) of radioactive calcium into the proventricus 785 or crop of laying hens, in order to estimate calcium absorption and/or the dynamics of body- 786 calcium compartments, including the bone. 787 Some of the other in vivo methodologies that were applied in mammals, even if they 788 provided information on the true or net absorption (in mass units), were not necessarily 789 completely suitable for use with birds. The balance method, although it provides estimations 790 of the actual absorption in mammals, cannot differentiate between renal and intestinal 791 excretion of Ca2+ in birds, whose urine and feces are both excreted via a single exit, the 792 cloaca. Therefore, in birds, the balance technique estimates the retention of the mineral rather 793 than its net or true absorption. Colostomy, followed by a balance study (Hurwitz et al. 1961), 794 enables feces and urine to be separated, and thus enables the determination of the true or net 27 795 Ca2+ absorption. However, the surgical treatment severely affects animal wellbeing, and often 796 interferes with normal egg laying, therefore, this technique, too, appears not to be completely 797 suitable for studies of reproducing female birds. Furthermore, as the balance method requires 798 steady-state conditions and relatively long sampling periods, this combined method appears 799 to be not fully adequate for detection of the changes in absorption during a single egg cycle. 800 The NARS technique overcomes these technical weaknesses and enables estimation of the 801 true absorption from the apparent mineral/NARS ratios in the diet and in the lower ileum. In 802 laying birds this technique provides the best estimations of the true absorption of Ca2+ or P 803 (Hurwitz et al. 1965; Hurwitz et al. 1966b; Hurwitz et al. 1973a; Bar et al. 1976a; Bar et al. 804 1978a; Cohen et al. 1978; Bar et al. 1984a) and other nutrients (Bielorai et al. 1973; Hurwitz 805 et al. 1973b)) in vivo. Data obtained with this technique confirmed many of the earlier 806 findings obtained in vitro or in situ (see 3.1.2, 3.1.3 and 3.3,2). 807 3.1.2. Site of absorption: In both mammalian and avian species, the proximal intestine 808 appears to have greater capability than the distal intestine to absorb Ca2+ in vivo (Wasserman 809 1962; Lengemann 1963; Cramer 1965; Hurwitz et al. 1967b; Pansu et al. 1983; Clunies et al. 810 1993), and it exhibits the most pronounced effect of vitamin D on Ca2+ absorption in vivo. 811 These in vivo findings were supported by in vitro studies (Schachter et al. 1959; Harrison et 812 al. 1960; Corradino 1973; Pansu et al. 1983). In mammals, despite the lack of TRPVs in 813 the distal intestine, the ileum is considered to be the major site for dietary calcium absorption, 814 most likely via a paracellular route (see 2.1, 3.2), because of the longer transit half-time 815 (reviewed in (Bronner 2003; Wasserman 2004)), and the lack of TRPVs. In birds, most of the 816 calcium is absorbed before it reaches the lower ileum, most likely as a result of the high 817 efficiency of the proximal intestine in absorbing Ca2+, and of the lower ileal electrochemical 818 potential difference (ECPD) (see 3.2, 3.3) (Hurwitz et al. 1966b; Hurwitz et al. 1972; Hurwitz 819 et al. 1973a). 820 3.1.3. Intestinal absorption of Ca2+ in birds, as influenced by nutrition or physiological 821 status: Vitamin D and its metabolites were found to be the most important factors regulating 822 intestinal Ca2+ absorption, both in growing non-laying birds (Migicovsky et al. 1951; 823 Wasserman 1962; Hurwitz et al. 1967b; Hurwitz et al. 1972; Corradino 1973) and in laying 824 birds (Table 4, (Hurwitz et al. 1972; Bar et al. 1976a; Cohen et al. 1978). This fundamental 825 fact was observed by means of in vitro, in situ and in vivo methodologies. Dietary alteration 826 of vitamin D metabolism caused by dietary calcium or P restrictions modulated the intestinal 827 absorption of Ca2+ (Nicolaysen 1943; Migicovsky et al. 1951; Wasserman 1962; Hurwitz et 828 al. 1965; Wasserman et al. 1968; Morrissey et al. 1971; Bar et al. 1973c; Hurwitz et al. 829 1973a; Bar et al. 1984a). The effect of dietary P restriction appears not to be fully dependent 830 on 1-hydroxylation of vitamin D3 in the kidney (Bar et al. 1973c; Bar et al. 1975b). Increased 28 831 dietary calcium diminished absorption capability (percentage absorption) but increased the 832 mass of absorption (Bar et al. 1979b). Some evidence suggests that the chemical or physical 833 form of dietary calcium may influence its absorption or retention in the growing chick 834 (Guinotte et al. 1991) or the laying hen (Chandramoni et al. 1998; Scheideler 1998; 835 Lichovnikova 2007). Some other evidence suggests that, as in the rat (Armbrecht et al. 836 1980a), also in the not laying birds, age, breed, rate of growth and sexual maturation (Wu et 837 al. 1994) or treatment with gonadal hormones (Nys et al. 1984c) may affect calcium 838 absorption. Whereas the effect of age on mammalian intestinal Ca2+ transport was indicated 839 directly in many studies ((Nicolaysen et al. 1953; Hansard et al. 1957; Armbrecht et al. 840 1980b) and others), the effect of age or rate of growth in non-laying birds is less obvious and 841 was indicated mostly indirectly (Bar et al. 1981a; Fox et al. 1981; Hurwitz et al. 1996). On 842 the other hand, in vivo studies of the laying hen indicated that there are also age-dependent 843 changes in Ca2+ absorption; however, these changes mostly result from the changes in Ca2+ 844 requirements associated with the changes in laying rate (Hurwitz et al. 1962; Scott et al. 845 1991). In addition, intestinal Ca2+ absorption is induced or diminished, respectively, by the 846 onset ((Hurwitz et al. 1973a; Bar et al. 1978a; Nys et al. 1980); reviewed in (Gilbert 1983)) or 847 arrest of egg laying (see 3.3.1). However, the intestinal capability to absorb Ca2+ did not reach 848 its maximum at the onset of production, but gradually increased during the early laying period 849 (Hurwitz et al. 1960; Scott et al. 1991). 850 3.2. Mechanisms of intestinal absorption 851 This issue was extensively reviewed in (Wasserman 1997; Bouillon et al. 2003; Bronner 852 2003; Wasserman 2004; Hoenderop et al. 2005b; Wasserman 2005; Perez et al. 2008; Khanal 853 et al 2008), among others. In birds, and mammals the relationship between luminal Ca2+ 854 concentration and Ca2+ absorption suggests that the overall absorption reflects a sum of 855 saturated and unsaturated processes. Whereas the saturated process corresponds to active 856 transcellular transport, the unsaturated process involves diffusion through a paracellular route. 857 Briefly, the transcellular mechanism, at least in birds, acts predominantly at the proximal 858 intestinal segments, the duodenum and jejunum. It consists of three major steps: entry of Ca2+ 859 through the brush border, facilitated diffusion or movement to the basal membrane, and 860 extrusion through the basal membrane. The first step proceeds down the chemical gradient of 861 Ca2+ that results from the low cellular concentration (10-7 M) and the high intestinal lumen 862 concentrations of Ca2+ from dietary sources (>10-3 M). This step appears to be facilitated by 863 TRPV6 (see 2.1.) and to a lesser extent by TRPV5. 864 The second step appears to be related to the presence of intestinal calbindins. Although 865 this relationship was elegantly visualized in 1990 by (Chandra et al. 1990) (see 3.1.1), it had 29 866 been suggested 24 years earlier (Wasserman et al. 1966) and was subsequently confirmed by 867 many other studies (Hurwitz et al. 1969b; Bar et al. 1972a; Freund et al. 1975). Kinetic 868 evidence supports the idea that intestinal calbindin facilitates Ca2+ absorption (Feher 1984; 869 Feher et al. 1992; Koster et al. 1995). In this regard, calbindin, may act as a transporter 870 protein (Feher 1984; Feher et al. 1992; Koster et al. 1995) or as a buffer that maintains the 871 chemical gradient of Ca2+ required for the undisturbed action of TRPVs (reviewed in 872 (Lambers et al. 2006b; Christakos et al. 2007; Schoeber et al. 2007). Some evidence also 873 indicates the occurrence of calbindin-mediated cytosolic-free diffusion of Ca2+ and of 874 vesicular transport of Ca2+ (reviewed in (Nemere et al. 1990; Norman et al. 2002; Larsson et 875 al. 2003; Dusso et al. 2005)). In addition to the hypothesized role of calbindins in Ca2+ 876 transport, they may also be involved in protecting the cells from high concentrations of Ca2+ 877 or from cellular degradation via apoptosis (Christakos et al. 2003). However, as the true or 878 net Ca2+ absorption in birds fed adequate P is positively related to its dietary intake, and is 879 negatively related to the intestinal rate of absorption or calbindin content (Fig. 2.) (Bar et al. 880 1979b), it appears that in the intestine calbindin acts as a transporter rather than as a buffer 881 protein. Otherwise, intestinal calbindin would be increased rather than decreased in birds fed 882 high dietary Ca2+. This hypothesis is somewhat weakened by the recent finding that 883 calbindin-D9K and TRPV6 are not required for vitamin D3-mediated Ca2+ absorption in the 884 mammalian small intestine (Akhter et al. 2007; Benn et al. 2008). These findings suggest that 885 1,25(OH)2D3 may affect intestinal absorption of Ca2+ via an alternative, the paracellular, 886 pathway. 887 The energy-dependent third step proceeds up the chemical gradient of Ca2+ that results 888 from the low cellular Ca2+ concentration (10-7 M) and the higher plasma Ca2+ concentration 889 (1.25 to 1.50 10-3 M). This step is facilitated by PMCA (see 2.3) and, to a lesser extent, by 890 NCXs (see 2.4). Components of all three steps of transcellular transport are vitamin D 891 dependent (see 2), because TRPV6 and TRPV5, calbindins and PMCA and, most likely, 892 Na+/Ca2+ exchanger genes all comprise a VDRE and/or are unaffected in the intestine of 893 VDR-knockout animals, and/or are stimulated in normal animals by 1,25(OH)2D3 or by 894 factors that affect its formation, such as dietary Ca2+, age, pregnancy, lactation, or egg laying. 895 The paracellular passive transport capability through the tight junctions occurs along 896 almost the whole length of the intestine, driven by the ECPD (Ussing 1949). As the electrical 897 potential difference (EPD) between blood plasma and the intestinal lumen is +5 to +15 mV 898 (blood always positive with respect to intestinal lumen), a chemical gradient is required in 899 order to maintain a positive ECPD between intestinal lumen and blood plasma. Such a 900 chemical gradient occurs in most animals that are fed adequate Ca2+ (Fig. 6). This suggests 30 901 that although the two mechanisms–paracellular and transcellular–appear to account for the 902 overall absorption, the paracellular transport is more important in animals fed adequate Ca2+. 903 904 905 906 907 908 Fig. 6. Relationship between the lumen-blood electrochemical potential difference (ECPD) of calcium, and calcium absorption in the duodenum (closed symbols) and jejunum (opened symbols) of laying hens fed diets containing 0.6 (circles), 1.8 (squares) and 4.0% calcium (triangulars). Calculated from data given in (Hurwitz et al. 1969b) 909 910 The possible involvement of 1,25(OH)2D3 in paracellular transport through the tight 911 junctions (TJ) is a matter of controversy. The TJ connects between epithelial cells and forms 912 a biological barrier. Some evidence supports the idea that paracellular transport is induced by 913 vitamin D (reviewed in (Wasserman 1997; Wasserman 2004)) or its active derivatives (Bar et 914 al. 1978b; Cohen et al. 1978). This evidence indicated, among other phenomena, that in birds 915 vitamin D affected the bidirectional flux in vivo (Hurwitz et al. 1972), and that 1- 916 hydroxylated derivatives of vitamin D3 affected Ca2+ absorption only during the period of 917 shell calcification, in spite the lack of change in calbindin content (Bar et al. 1976b; Cohen et 918 al. 1978) (see also 3.3.2.1). Other related phenomena observed in mammals were reviewed in 919 (Wasserman 2005). Some other publications, dealing with non-laying animals ((Nellans et al. 920 1978; McCormick 2002) and others; reviewed in (Wasserman 2004)) oppose this hypothesis. 31 921 More recent studies supported the idea that the tight junction (TJ) proteins, permeability 922 and paracellular transport (reviewed in (Schneeberger et al. 2004; Van Itallie et al. 2006)) 923 seem to be controllable. It appears that 1,25(OH)2D3 and VDR, as well as cellular Ca2+, are 924 among the factors responsible for preserving the integrity of TJ complexes, up-regulating TJ 925 proteins, and modulating TJ permeability and paracellular Ca2+ transport (Tang et al. 2003; 926 Kutuzova et al. 2004; Fujita et al. 2008; Kong et al. 2008). These findings further support the 927 hypothesis that 1,25(OH)2D3 may regulate paracellular Ca2+ transport also. 928 3.3. Absorption of Ca2+ in the laying bird 929 3.3.1. Development of absorption capability: The impressive intestinal Ca2+ absorption 930 capability of the laying bird is built up, in parallel with the increased demands for Ca2+ during 931 growth, maturation and onset of reproduction (Fig. 7, Table 4). Accelerated growth (Bar et al. 932 1981a; Hurwitz et al. 1995; Bar et al. 2003) increased the Ca2+ flux from blood to bone. The 933 growth rate, in turn, is controlled by genetic, nutritional, environmental and zootechnical 934 factors, and involves growth, thyroid and other hormones. Detection of homeostatic error 935 (i.e., change) (Hurwitz 1996; Ramasamy 2006; Bar 2008) in blood plasma Ca2+ level elicits 936 enhanced PTH secretion and, consequently, vitamin D metabolism, and acceleration of the 937 three presently known mechanisms associated with the active transcellular absorption of Ca2+. 938 Passive paracellular transport of Ca2+ is, most likely, also stimulated by vitamin D (see 3.2). 939 Later, following attainment of a certain minimal BW, light stimulation induces the synthesis 940 and activity of hypothalamic-pituitaric stimulating hormones and, consequently, the onset of 941 gonadal activity. As a result, estrogen/androgen-dependent new medullary bone and plasma 942 Ca2+ binding proteins (synthesized in the liver) are formed. All of these processes drain Ca2+ 943 from the extra-cellular fluids and induce further vitamin D metabolism, either directly or 944 through PTH secretion. During this stage of maturation the gonadal activity stimulates the 945 development of the oviduct (including the ESG). Finally, the onset of laying of calcified eggs 946 imposes severe extra demands upon Ca2+ homeostasis, resulting in further induction of 947 vitamin-D-dependent intestinal Ca2+ absorption, especially during the period of shell 948 calcification. The intestinal capability to absorb Ca2+ does not reach its maximum at the onset 949 of laying, but continues to increase gradually during the early laying period (Hurwitz et al. 950 1960; Scott et al. 1991). 951 32 952 953 954 955 956 957 958 959 960 961 962 963 964 965 966 Fig. 7. Development of the intestinal and eggshell gland (ESG) Ca 2+ transport capabilities in the laying bird. First phase - growth, white rectangular and arrows; second phase - maturation, gray (no line around) rectangular and arrows; third phase - laying, black rectangular and arrows. For details see text (2, 3.3.1). Components of the transport mechanisms are printed in italic fonts. In parentheses are listed components of intestinal paracellular transport not yet found to be directly dependent on vitamin D (dependency is characterized either by the responsiveness to vitamin D metabolites, the specific appearance in vitamin D receptor-knockout animals or, in the case of proteins, also by the presence of a vitamin D-responsive element on their encoding gene), but may be dependent on ESG CA. Ca, calcium; CAs, carbonic anhydrases; ECPD, electrochemical potential difference; EPD, electrical potential difference; NCXs, Na+/Ca2+ exchangers; PMCAs, Ca2+ATPases; OPN, osteopontin; TJ, tight junction proteins; TRPVs, epithelial calcium channels. A single question mark indicates that the presence or involvement of this component of transport was not yet determined in birds, whereas a pair of question marks indicates that most of the available evidence does not support the idea that vitamin D regulates ESG Ca 2+ transport, although such an idea cannot be completely rejected. 33 967 3.3.2. Circadian calcium absorption: The most remarkable finding concerning Ca2+ 968 absorption in the laying bird is that it is noticeably more intense during the period of shell 969 calcification than during periods of ESG inactivity (Table 4; and (Hurwitz et al. 1965; 970 Hurwitz et al. 1966b; Hurwitz et al. 1973a; Bar et al. 1976a; Bar et al. 1978a; Cohen et al. 971 1978; Bar et al. 1984a)). Whereas the other nutritional and physiological responses were 972 observed in situ and in vivo, the effect of shell calcification was observed only by means of 973 the NARS techniques (see 3.1.1), with the aid of 974 dioxide, but not polyethylene glycol ((Hurwitz et al. 1965; Bar et al. 1979b; Sunahra et al. 975 1989; Waddington et al. 1989) and unpublished results). However, this finding was supported 976 by the kinetic study of (Clunies et al. 1993) and by the study of (Watanabe et al. 1992) on the 977 venous: arterial (duodenal: carotis) ratios of phosphorus, total calcium and Ca2+ , as well as by 978 the higher daily net absorption of Ca2+, observed by the balance technique, on laying days than in 979 non-laying days (Taylor and Kirkley, 1967). On the other hand, the effect of shell formation on 980 Ca2+ absorption capability was not confirmed by application of the in situ loops methods 981 (Wasserman et al. 1978; Nys et al. 1980). This discrepancy could have arisen because the 982 medium composition in the in situ studies did not reflect the lumen content in vivo, especially 983 with regard to the activity or solubility of Ca2+ (Hurwitz et al. 1969b) and their variations 984 during the egg cycle (Nys et al. 1980) (see 3.3.2.2 and 3.3.2.3). 91 Y, 144 Ce, chromic oxide or titanium 985 Whereas the combined transcellular and paracellular transport during the period of shell 986 formation account for net absorption of about 80 and 65% of the dietary Ca2+ intake in hens 987 and quail, respectively, much less (36 and 18%, respectively) is absorbed during the period of 988 ESG inactivity. Non-laying hens and quail absorbed slightly less (23 and 17%, respectively) 989 (Hurwitz et al. 1969a; Hurwitz et al. 1973a; Bar et al. 1976a; Bar et al. 1976b). 990 The increased calcium absorption during shell formation corresponds to the decreases in 991 plasma Ca2+ and the increased plasma PTH (Dacke 1976; van de Velde et al. 1984; Singh et 992 al. 1986), the increased intestinal content of soluble calcium (Mongin 1976b; Mongin 1976a), 993 acidosis (Mongin et al. 1964; Cohen et al. 1974; Wideman et al. 1985) and the changes in 994 plasma content of the reproductive hormones during the ovulatory cycle (reviewed in (Etches 995 1996)). 996 3.3.2.1. The vitamin D dependency: The mechanism of the circadian variation in Ca2+ 997 absorption in the laying domestic bird is not yet clear. As mentioned above, it appears to be 998 sensitive to vitamin D; in vitamin-D-fed birds, an exogenous supply of 1-hydroxylated 999 derivatives of vitamin D markedly affected Ca2+ and P absorption during shell formation, 1000 whereas its effect during the period of ESG inactivity (when shell was not being formed) was 1001 small or negligible (Bar et al. 1976b; Cohen et al. 1978). The fact that shell formation affects 34 1002 Ca2+ absorption in birds fed vitamin D3, as well as in those fed 1OHD3 suggests that the 1003 increased absorption is not dependent on 1-hydroxylation in the kidney. This idea is 1004 supported by our earlier findings on the lack of considerable enhancement of renal 1- 1005 hydroxylase activity or on the plasma content of 1,25(OH)2D3 (Table 2b; reviewed in (Bar 1006 2008), and it was even suggested that 1,25(OH)2D3 production decreased during shell 1007 calcification (Kenny 1976)). Some findings did indicate diurnal changes in vitamin D 1008 metabolism (reviewed in (Bar 2008)), but as the build-up of genomic effects of 1,25(OH)2D3 1009 (metabolism, transcription and translation; (Lawson 1978; Bar et al. 1992a)) requires several 1010 hours, the circadian changes in calcium absorption during the relatively short egg cycle could 1011 not be attributed to any circadian change in vitamin D metabolism. Nevertheless, the increase 1012 in absorption during shell formation could not be mediated through calbindin, which 1013 remained almost unchanged during the laying cycle (Bar et al. 1972a; Bar et al. 1975a; Bar et 1014 al. 1976a; Cohen et al. 1978; Wasserman et al. 1978; Nys et al. 1992a; Ieda et al. 1995; Goto 1015 et al. 2002a). As the available data also do not indicate the occurrence of circadian changes in 1016 the other components of the transcellular mechanism, the hypothesis that, during shell 1017 formation in vitamin-D-fed birds, vitamin D interferes with the paracellular, rather than with 1018 the transcellular transport, is further supported. 1019 The enhanced plasma PTH concentration during shell formation (Table 2b) does not 1020 appear to be associated with an immediate vitamin D-dependent effect on Ca2+ absorption, 1021 because of the time required for vitamin D metabolism to be expressed (Lawson 1978; Bar et 1022 al. 1992). In addition, temporarily high levels of circulating PTH have not been found to 1023 affect temporary intestinal Ca2+ absorption, with the exception of a very few cases (reviewed 1024 in (Nemere et al. 2002)). 1025 3.3.2.2. Acidosis: Mammalian nutritional acidosis, but not environmental acidosis, was 1026 widely studied and the findings on its effects on intestinal calcium absorption were 1027 inconsistent, and have aroused controversy (Gafter et al. 1980; Goulding et al. 1984; Favus et 1028 al. 1986). Recently (Charoenphandhu et al. 2006), who used the rat as a model animal, 1029 suggested that chronic metabolic acidosis acts on TJ proteins and stimulates the paracellular 1030 transport. In addition, it stimulates the expression of TRPV6 and PMBA1b, which are known 1031 to be involved in active intestinal transcellular transport of calcium (see 3.2). 1032 The direct effect of acidosis on calcium absorption in laying birds was not determined. In 1033 the laying chicken, which calcifies shell mainly during the dark period of the day, acidosis 1034 occurs in close temporal association with the changes in calcium absorption and shell 1035 calcification that take place during the shell cycle. This indicates a possible involvement of 1036 acidosis in the cyclic changes in calcium absorption capability, through its effect on intestinal 35 1037 solubility of Ca2+ (Mongin 1976b; Mongin 1976a). This hypothesis is somewhat weakened by 1038 the finding that in laying quail, which calcified shells mostly during the light period, acidosis 1039 reached a peak much earlier than maximal shell calcification (Dacke et al. 1973) and maximal 1040 calcium absorption. 1041 3.3.2.3. Electrochemical potential difference (ECPD): In chicken and quail, both laying and 1042 non-laying, that are fed adequate Ca2+ a high positive ECPDs between the intestinal lumen 1043 and the blood were established in the duodenum, jejunum and proximal ileum, but not the 1044 distal ileum (Hurwitz et al. 1968; Hurwitz et al. 1969b). Thus, these birds appear to utilize the 1045 paracellular as well as the transcellular transport pathways. The lower ECPD in the distal 1046 intestinal segments of the chicken prevents absorption of significant quantities of Ca2+ in the 1047 distal ileum, and ensures that in chicken and quail, unlike mammals, Ca2+ is almost entirely 1048 absorbed before it reaches the distal ileum (Hurwitz et al. 1965; Hurwitz et al. 1972; Hurwitz 1049 et al. 1973a; Bar et al. 1976a). When the normal diets of the domestic birds contain more than 1050 0.6% Ca2+ the ECPD is maintained high enough to drive Ca2+ transport from blood to the 1051 intestinal lumen, and the paracellular route of transport appears to predominate. This 1052 hypothesis is supported by the finding that the vitamin-D-induced component of the 1053 transcellular pathway is down-regulated when dietary Ca2+ is increased (see 2, 3.2), which 1054 indicates that the transcellular pathway therefore contributes little to the overall absorption. 1055 The small change in plasma Ca2+, that is associated with shell calcification, together with 1056 the three- to fourfold increase in intestinal soluble calcium concentration (Mongin 1976b; 1057 Mongin 1976a) during shell formation is enough to affect the ECPD markedly and, 1058 consequently, to increase the passive Ca2+ absorption noticeably. Such increased absorption 1059 could also be promoted by any other change in intestinal lumen Ca2+ concentration that may 1060 result from dietary P deficiency, high dietary calcium intake, or low water intake; it also 1061 could be affected by changes in intestinal pH or by the diminution in the intestinal content 1062 during darkness. All of these factors combine to affect the intestinal calcium to P 1063 interrelationships. Recalculation of the data given in (Hurwitz et al. 1971) suggests that, 1064 whereas the total calcium to P ratio remains quite constant (ranging from 1.52 to 1.69) along 1065 the intestine, the ultrafilterable-calcium:P ratio increased from 1.73 in the duodenum to 12.1 1066 in the lower ileum. This suggests that absorption of P is faster than that of Ca2+, so that 1067 relatively more Ca2+ is left in the intestinal lumen, where it affects ECPD and paracellular 1068 transport. 1069 The involvement of vitamin D in the regulation of paracellular transport of Ca2+ is a matter 1070 of controversy. Our studies with the laying hen support the hypothesis that 1,25(OH)2D3 also 1071 affects paracellular transport: the specific, but notable, effect of 1-hydroxylated vitamin D 1072 metabolites on the absorption of Ca2+ through the paracellular route in the laying bird occurs 36 1073 exclusively during eggshell formation, although the calbindin (Bar et al. 1978b; Cohen et al. 1074 1978) and, most likely, the PMCA (see 2.3) concentrations remained unchanged during the 1075 egg cycle. Only when dietary calcium was lower than 0.6% and the ECPD became negative 1076 or too low, 1,25(OH)2D3 synthesis was up-regulated and the transcellular transport appeared 1077 to become the main mechanism. 1078 3.3.3. Bone–the other source of shell calcium: The increased absorption during shell 1079 calcification is not sufficient to satisfy the high Ca2+ requirement for shell calcification. In 1080 light of the passage times (50% output) of the ingested food through the esophagus (3 h), 1081 stomach (1 h), and the whole intestine (3 to 4 h) (Hurwitz et al. 1966b), and of the fact that 1082 most of the Ca2+ is absorbed in the upper intestine (Hurwitz et al. 1965; Hurwitz et al. 1973a; 1083 Bar et al. 1976a) during the first 75 to 78 min following its leaving the stomach, it is evident 1084 that, 6 to 10 h after the interruption of feeding, the intestine of the laying bird is almost empty 1085 of Ca2+. As eating stops with the onset of darkness, and as shell calcification in the domestic 1086 hen occurs mostly during the dark period (Tables 1), a considerable proportion of the shell 1087 Ca2+ must be derived from bone Ca2+ reserves. A conservative, careful estimate of this 1088 proportion is 20 to 40% of the eggshell Ca2+ (Comar et al. 1949; Driggers et al. 1949; Clunies 1089 et al. 1993). The bone reservoir is subsequently replenished with Ca2+ from the intestinal 1090 source during the periods of ESG inactivity and "plumping". Thus, in the high-producing 1091 domestic birds most of the shell Ca2+ of each individual egg is derived daily from the 1092 intestine. The resulting calcium balance [intake - (urine + shell + feces)] in the domestic hen 1093 varied between +80 and -121 mg/d (Hurwitz et al. 1960; Hurwitz et al. 1969a; Waddington et 1094 al. 1989; Clunies et al. 1993). Considering the fact that balance studies usually continue over 1095 3 to 7 d and that shell is not formed during some of these days (depending on the rate of 1096 laying), the balance may be even smaller on the days when shell is formed. Therefore, the 1097 calcium content in successive eggs in the clutch tends to decline progressively, especially in 1098 hens with long clutches, in old ones, or in those characterized by thin shells (Bar et al. 1988; 1099 Bar et al. 1999). The quail appears to be better adapted than the chicken with regard to shell 1100 calcification; it can obtain a higher proportion of shell Ca2+ from dietary source because it 1101 tends to oviposit at the end of the light period (reviewed in (Dacke 1979) and therefore 1102 calcifies eggs while the intestinal lumen contains enough Ca2+. 1103 37 1104 4. Eggshell transport of calcium 1105 4.1. Early studies on the evaluation of the capability and regulation of calcium transport 1106 in the eggshell gland 1107 Variables related to shell quality and shell mass have been widely employed for the 1108 estimation of ESG calcium transport in vivo. In fact such estimations reflect the accumulated 1109 contribution of all the mechanisms involved in shell formation (such as calcium homeostasis, 1110 acid/base balance, vitamin D metabolism, intestinal absorption, bone resorption and ESG 1111 activities), rather than the particular mechanism of ESG calcium transport. Furthermore, 1112 unlike the intestinal methodologies in vivo, the ESG ones were inadequate for the estimation 1113 of the ESG capability to transport Ca2+. More specific estimations were obtained during the 1114 7th and 8th decades of the 20th century (Ehrenspeck et al. 1967; Ehrenspeck et al. 1971; 1115 Pearson et al. 1973; Pearson et al. 1974; Pearson et al. 1977; Eastin et al. 1978b; Eastin et al. 1116 1978a). The most comprehensive studies were those performed by Eastin and Spaziani and by 1117 Pearson et al. Little was done since then (Nakada 1990; Vetter et al. 2005). Additional 1118 information on this issue was obtained from other reports that focused on the ESG fluid 1119 content (el Jack et al. 1967; Rieser et al. 1972; Edwards 1977; Arad et al. 1989; Nakada et al. 1120 1990; Nys et al. 1991; Panheleux et al. 1999), ESG electrical activity (Hurwitz et al. 1970; 1121 Cohen et al. 1973; Shimada et al. 1986), and on the activities and/or expression of proteins 1122 believed to be involved in shell formation (see 2, 4.2). 1123 The methodologies tried in seeking for the mechanisms and regulation of ESG Ca2+ 1124 transport were: isolated ESG tissue in vitro (Ehrenspeck et al. 1971; Pearson et al. 1973; 1125 Laklia 1981; Vetter et al. 2005); use of Ussing (Ussing et al. 1951) type apparatus; ESG 1126 perfusion in situ (Eastin et al. 1978a; Eastin et al. 1978b); kinetic evaluation using radio- 1127 labeled calcium administered either as single doses or as continuously administration (Comar 1128 et al. 1949; Hurwitz 1964; Clunies et al. 1993); and determination of the venous:arterial 1129 (uterine:carotis) ratios of phosphorus, total calcium and Ca2+ contents (Watanabe et al. 1992). 1130 The major findings of these and other (Nakada 1990; Nakada et al. 1990) studies were: (a) 1131 more Ca2+ was transported in the ESG than in the other regions of the oviduct (Eastin et al. 1132 1978a; Laklia 1981); (b) non-laying or molting birds had a lower calcium transport (secretion 1133 from serosa or plasma to mucosa or lumen) and lower CA activity (Pearson et al. 1977; Eastin 1134 et al. 1978a) than laying birds; (c) Ca2+ secretion increased during the period of shell 1135 formation (Ehrenspeck et al. 1967; el Jack et al. 1967; Rieser et al. 1972; Nakada 1990; 1136 Watanabe et al. 1992; Clunies et al. 1993), and increased further if an egg or an egg-like 1137 insertion was present in the ESG (Edwards 1977; Eastin et al. 1978a; Nakada et al. 1990); (d) 1138 the EPD (plasma to lumen) was more positive in shell-forming hens (Hurwitz et al. 1970; 38 1139 Cohen et al. 1973); (e) The EPD favored the secretion of K+, Ca2+, and lumen-to-plasma 1140 transport of Cl-, but not the plasma-to-lumen flux of HCO3- or the lumen-to-plasma flux of 1141 Na+ (Pearson et al. 1974; Eastin et al. 1978b), except that at luminal Ca2+ contents of 6 to 8 1142 mM the ECPD would became negative (plasma-to-lumen) and then Ca2+ passive diffusion 1143 would be arrested; (f) however, Ca2+ secretion occurred even in the absence of EPD 1144 (Ehrenspeck et al. 1971; Pearson et al. 1973); (g) Ca2+ transport and CA activity appeared to 1145 be dependent on oxidative metabolism and on the active transport of Na+ (lumen to plasma) 1146 (Ehrenspeck et al. 1967; Ehrenspeck et al. 1971; Pearson et al. 1974; Vetter et al. 2005); (h) 1147 Ca2+ secretion was unaffected (Pearson et al. 1973), or continued, even if at a reduced level, 1148 when luminal Na+ was low or when its transport was prevented by the presence of ouabain 1149 (an Na+ transport inhibitor) (Pearson et al. 1974); (i) the CA inhibitor acetazolamide markedly 1150 affected CA activity (Eastin et al. 1978a; Lundholm 1990) and net Ca2+ transport in vitro, in 1151 situ (Pearson et al. 1974; Pearson et al. 1977; Eastin et al. 1978b; Laklia 1981) and in vivo 1152 (Bernstein et al. 1968; Sauveur et al. 1978; Bar et al. 1992a) (see also 2.5). On the other hand, 1153 (Ehrenspeck et al. 1971) found indications of increased permeability to Ca2+ in 1154 acetazolamide-treated ESG tissue in vitro; (j) the effect of acetazolamide was not observed in 1155 non-laying or molting hens (Pearson et al. 1977); (k) Ca2+ secretion was affected by luminal 1156 HCO3- but less affected by luminal Ca2+, whereas HCO3- movement depended mostly on its 1157 luminal concentration (Eastin et al. 1978b). 1158 4.2. Eggshell gland cyclic functionality 1159 The transport of Ca2+ and its complementary anion HCO3- by the ESG fluctuates during 1160 the egg-formation cycle, and both peak during the second half of the cycle. More specifically, 1161 the peak occurs during the later stage, beginning 11 to 12 h post ovulation and ending 22 to 1162 24 h post ovulation. Unlike its transport in the intestine, Ca2+ transport in the ESG is not 1163 associated with massive transport of many other nutrients, although it is associated with the 1164 secretion of HCO3- and the counter-transport of Na+, Cl- and H+ (Nys et al. 1999; Vetter et al. 1165 2005), and with the secretion of minute masses of shell matrix proteins. The development of 1166 the capabilities to transport Ca2+ and the other ions is associated with morphological and 1167 functional development of the oviduct, and is triggered by gonadal hormone activities, 1168 especially of estrogens (Oka et al. 1969; Navickis et al. 1979b; Hora et al. 1986; Bar et al. 1169 1990b; Striem 1990), during sexual maturation and onset of egg production. Estrogens appear 1170 also to maintain oviductal integrity and functionality through their anti-apoptotic capability 1171 (Monroe et al. 2002), as was clearly demonstrated during molt induction (Yoshimura et al. 1172 1997; Braw-Tal et al. 2004; Anish et al. 2008). 39 1173 In contrast to the situation in the intestine, Ca2+ homeostasis and vitamin D metabolism 1174 have no specific or major role (Navickis et al. 1979b; Striem et al. 1991; Corradino et al. 1175 1993) in the development of ESG capacity to transport Ca2+. 1176 1177 4.3. Shell-gland-specific proteins 1178 Many of the egg-membrane, shell-matrix and ESG proteins (reviewed in (Solomon 1991; 1179 Nys et al. 1999; Johnson 2000; Arias et al. 2001; Nys et al. 2001; Soledad Fernandez et al. 1180 2001)) are formed specifically in the ESG or are present there in high concentrations. Some 1181 of them (including collagen and many others) appear to be structural proteins, whereas others 1182 (including CA, calbindin, CaATPase, Na+-K+ATPase, parathyroid hormone-related peptide 1183 (PTHRP), VDR and other receptors) reveal specific functionality or appear to have both 1184 structural and functional characteristics (as with OPN or dermatan sulfate). 1185 Several of the ESG proteins, (such as calbindin (see 2.2), nVDR (Ieda et al. 1995), 1186 estrogen receptors (ER) (Kawashima et al. 1985), progesterone receptors (PR) (Kawashima et 1187 al. 1982), PTHRP (Thiede et al. 1991), glypican-4, 1-Na+-K+-ATPase (Lavelin et al. 2001; 1188 Lavelin et al. 2002) and OPN (Pines et al. 1995)) are transcribed in a circadian manner. 1189 Whereas calbindin, VDR and 1-Na+-K+-ATPase (in the glandular epithelium) are stimulated 1190 by Ca2+ transport (Bar et al. 1973b; Nys et al. 1989; Striem et al. 1991; Ieda et al. 1995; 1191 Lavelin et al. 2001; Goto et al. 2002c), a few others, e.g., OPN, 1-Na+-K+-ATPase (in the 1192 pseudostratified epithelium) and glypican-4 are stimulated by the mechanical strain imposed 1193 by the forming egg (Lavelin et al. 1998; Lavelin et al. 2001; Lavelin et al. 2002). The 1194 circadian transcription does not necessarily account for the circadian functionality. On the 1195 assumption that the differences between the kinetic rates of synthesis and of degradation of 1196 the fluctuating proteins and their mRNAs in the ESG are similar to those observed for 1197 calbindin, i.e., about one order of magnitude (Bar et al. 1992a), the fluctuations in the 1198 mRNAs would not be expected to cause significant fluctuations in the protein concentrations 1199 and, therefore, would not be relevant to the daily changes in functionalism–at least not during 1200 a single clutch of more than three eggs. However, prolongation of the intervals between 1201 successive eggs, i.e., at the ends of clutches, for more than 20 h may markedly reduce 1202 concentrations of these proteins as previously shown for calbindin (Montecuccoli et al. 1203 1977b). 1204 Eggshell calcification, which occurs in the ESG requires the interaction of many 1205 processes. These include: transcellular and/or paracellular transport of Ca 2+, the transfer of 1206 other ions and water during the early phase of egg presence in the ESG, when ovalbumin (egg 40 1207 white) swelling ("plumping") occurs, as well as during the later phase of shell calcification; 1208 egg movement along the oviduct, and oviposition driven by oviductal tissue contractions and 1209 their regulation; secretion of shell matrix proteins; and recognition by receptors of the many 1210 hormones involved in oviduct development and functionality. Whereas the receptors of the 1211 gonad hormones are found in higher concentrations in the ESG (Turner et al. 1978; Tanaka 1212 1980; Kawashima et al. 1982) than in the intestine (Agemori et al. 1984), the VDR content of 1213 the ESG appears to be markedly lower than that of the intestine (by a factor of three to seven) 1214 (Coty 1980; Bar et al. 1984b). Despite the lower VDR content of the ESG, this organ is 1215 characterized by relatively high concentrations of vitamin-D-dependent proteins, i.e., proteins 1216 encoded by genes known to have a VDRE, or vitamin-D-induced proteins, including: CA, 1217 calbindin, CaATPase (see 2), VDR itself, and OPN (Prince et al. 1987; Noda et al. 1990). 1218 However, unlike the well-documented vitamin D dependency of the intestinal absorption of 1219 Ca2+, the vitamin D dependency of ESG transport of Ca2+ is not certain. This uncertainty, in 1220 part, results from the difficulties of distinguishing between a possible specific effect of 1221 vitamin D on the shell calcification, on the one hand, and, on the other hand, an indirect effect 1222 induced by egg formation, since every factor that impairs or delays egg laying will 1223 consequently indirectly inhibit vitamin D metabolism and expression (reviewed in (Bar 1224 2008)), because of the decline in physiological Ca2+ demands. The apparent lack of an effect 1225 of vitamin D on ESG transport of Ca2+ is supported by the finding (Striem et al. 1991) that 1226 laying quail fed a vitamin-D-deficient, high-calcium diet exhibited all the symptoms typical 1227 of vitamin D-deficiency in the intestine, bone and blood, but continued to lay partially 1228 calcified eggs and to maintain for a long period moderate contents of the vitamin-D- 1229 dependent protein, calbindin and its mRNAs. 1230 4.4. Mechanism of calcium transport in the eggshell gland 1231 The available data appear to support the hypothesis that calcium secretion into the ESG 1232 lumen, similarly to the intestinal absorption process, occurs by both active transport and 1233 passive diffusion (Eastin et al. 1978a; Eastin et al. 1978b). Two of the three components 1234 involved in transcellular intestinal Ca2+ transport, i.e., calbindin and Ca2+ATPase, are found 1235 in the ESG and in the distal isthmus (Corradino et al. 1968; Eastin et al. 1978a; Eastin et al. 1236 1978b; Wasserman et al. 1991) tissues. Their presence in the distal isthmus suggests that 1237 there is active Ca2+ transport in this region, which is also known to secrete the mammillae 1238 where the early seeding of the eggshell Ca2+ crystals is initiated. In the ESG, calbindin is 1239 localized mainly in the tubular gland that is considered to be the major origin of eggshell Ca2+ 1240 (Wasserman et al. 1991). The Ca2+ATPase is localized primarily in the apical-microvillar 1241 membranes of the same tubular gland cells (Yamamoto et al. 1985; Wasserman et al. 1991), 41 1242 facing the ESG lumen where shell calcification occurs. Thus, although the presence in the 1243 ESG of the third component of active/transcellular Ca2+ transport, TRPVs, has not yet been 1244 confirmed (see 2.1), it is most likely that TRPVs (also found in the mammalian uterus) are 1245 present there, and that transcellular transport is involved in eggshell calcification. 1246 Many studies (el Jack et al. 1967; Mongin et al. 1970; Rieser et al. 1972; Edwards 1977; 1247 Arad et al. 1989; Nakada et al. 1990; Nys et al. 1991; Panheleux et al. 1999) indicated an 1248 increase in ESG luminal content of Ca2+ during shell calcification. This suggests that there 1249 was an apparent "uphill" transport of Ca2+ and supports the hypothesis that a major portion of 1250 the secretion of Ca2+ into the ESG lumen occurs against the ECPD and involves active 1251 transport. However, the positive (plasma with respect to mucosa) electrical potential 1252 difference (EPD) (Hurwitz et al. 1970; Cohen et al. 1973; Pearson et al. 1973; Eastin et al. 1253 1978a) during shell calcification provides a sufficient electromotive force to ensure the 1254 transport of Ca2+ from blood to lumen against the chemical gradient, and to maintain a 1255 “steady state” calcium activity in the ESG that is over twice (Hurwitz et al. 1970) to four 1256 times (Eastin et al. 1978b) as high as that in the blood plasma. Furthermore, whereas the 1257 elevation in ESG luminal calcium during the period of shell calcification was demonstrated in 1258 several studies, the actual in vivo luminal content of ESG Ca2+, and its distribution in the 1259 lumen are less certain. Only a few studies measured ionic calcium, rather than total or 1260 diffusible calcium, in the ESG fluid (Nys et al. 1991), and all determinations were performed 1261 on uterine fluid dripped out of the vagina following artificial egg expulsion. The exposure of 1262 the fluid to the external atmosphere prior to the analysis, as well as the artificial (hormonal or 1263 mechanical) nature of the egg expulsion put some doubt on the accuracy of the observed 1264 values of ESG Ca2+. The ECPD-facilitated transport theory assumes the existence of a quite 1265 simple system consisting of a biological "membrane(s)" separating two or more well-mixed 1266 compartments. This is not necessarily the case in the calcifying ESG, where the "membrane" 1267 is composed of a variety of cells, many of which (the glandular cells) are loaded with either 1268 free or bound calcium, and that may consist of one or more compartments. Furthermore, there 1269 is also uncertainty regarding the Ca2+ concentration in the luminal zone adjacent to the Ca2+- 1270 secreting cells. It is most likely that Ca2+ is not uniformly distributed, and that its 1271 concentration changes rapidly in response to CA activity and shell calcium-carbonate 1272 formation. The latter acts as a "sink" for the secreted Ca2+ and may create a luminal Ca2+ 1273 gradient within the ESG lumen that may ensure a blood-to-lumen chemical gradient 1274 facilitating the passive diffusion of Ca2+. This, together with the markedly lower relative 1275 concentration of VDR in the ESG, and the accumulated evidence for the lack of a direct 1276 major effect of vitamin D on the ESG components of transcellular Ca2+ transport, especially 42 1277 on calbindin transport (see 2.2), suggest that the active, vitamin-D-dependent transcellular 1278 transport of Ca2+ plays only a partial role in eggshell calcification. The remaining transported 1279 Ca2+ is likely to be obtained through passive paracellular mechanisms, as occurs in the 1280 intestine. Wasserman (Wasserman et al. 1991) speculated that the epithelial lining cells are 1281 those responsible for the diffusion path of Ca2+ secretion. This path utilizes both the EPD 1282 gradient (Hurwitz et al. 1970; Cohen et al. 1973; Pearson et al. 1973; Eastin et al. 1978a) and 1283 the rapid removal of the transported Ca2+ from the lumen fluid, by binding to HCO3-. 1284 The accumulated evidence suggests that HCO3- may be more important for shell 1285 formation than previously believed, and that it could be the major driving force for Ca2+ 1286 transport. The evidence includes the strong association of the active secretion of HCO3- in the 1287 ESG with the transport of Cl-, active transport of Na+, and activity and expression of Na+- 1288 K+ATPase (Eastin et al. 1978a; Eastin et al. 1978b; Lavelin et al. 2001; Vetter et al. 2005). 1289 All these phenomena in the laying hen are affected by age and molt, or are associated with the 1290 egg cycle. The speculation that CA is the driving force for Ca2+ deposition is further 1291 supported by some of the observations mentioned above (see 4.1). These included: continued 1292 Ca2+ secretion when luminal Na+ was low or while its transport was prevented; the critical 1293 effects of the CA inhibitor, acetazolamide, on ESG Ca2+ transport; the lack of an effect of 1294 acetazolamide on non-laying or molting hens; and the determinant effects in situ of luminal 1295 HCO3- on Ca2+ secretion and HCO3- movement as compared with the effect of luminal Ca2+ 1296 (Pearson et al. 1977; Eastin et al. 1978a; Mongin 1978; Nys et al. 1984a; Lundholm 1990; 1297 Bar et al. 1992a), whereas in vitro HCO3- affects only CA activity (see also 2.5). If this 1298 speculation is valid, then the apparently active overall CaCO3 deposition actually comprises 1299 the active transport/formation of HCO3- followed by an active-like passive transport of Ca2+ 1300 along the EPD. The rapid removal of Ca2+ from the ESG lumen and its deposition in the 1301 eggshell calcium crystals would further enhance this mechanism. This hypothesis, however, 1302 has not yet been confirmed, nor does it exclude the possible involvement of transcellular 1303 transport as the major or a complementary pathway of Ca2+ transport in the ESG. 1304 5. Conclusions and speculations 1305 The available data are insufficient to support a firm hypothesis regarding the overall 1306 mechanism of calcium transport in the laying bird, i.e., the presented hypothesis is somewhat 1307 speculative. This, together with the prospects of a further drop in research on avian 1308 physiology, highlights the urgency of the need to address the uncompleted hypothesis or to 1309 offer rational speculations, at least as further working hypotheses (Fig. 8). 43 1310 1311 1312 1313 1314 1315 1316 1317 1318 1319 1320 Fig. 8. Mechanisms and factors associated with the transfer of Ca2+ from the intestine to the eggshell. The overall phenomena involved three major transporting systems: (a) intestinal net Ca2+ absorption; (b) eggshell gland (ESG) net secretion of Ca2+ and HCO3-; and (c) bone (medullary) remodeling. These mechanisms are regulated by a variety of metabolic, endocrine and neurological factors (for details see text and (Etches 1996; Nys et al. 1999; Dacke 2000; Sugiyama et al. 2001; Sharp 2005; Bar 2008; Khanal et al. 2008). Abbreviations: EST, estrogens; PR, progesterone; PG, prostaglandins; PTG, parathyroid gland; PTH, parathyroid hormone; 1,25-D3, 1,25-dihydroxy vitamin D3; CA, carbonic anhydrase; MEDUL., medullary; PBP, plasma-calcium binding proteins. Above the dotted black line are given the events associated with sexual (marked in the figure with numbers within parentheses: #: 1,2,4,5,10-15,18-21) 44 1321 1322 1323 1324 1325 1326 1327 1328 1329 1330 1331 1332 1333 1334 1335 1336 1337 1338 1339 1340 1341 1342 1343 1344 1345 1346 1347 1348 1349 1350 1351 maturation and the early stages of egg formation and plumping (marked in the figure with numbers within parentheses: #: 2-5,8-10,12-14,16?,18,19?20,22,23); below it are given those associated with calcification (marked in the figure with numbers within parentheses: #: 2,4,6-22,24-26). Light- and dark-gray frames represent transporting and endocrine organs, respectively; black arrows represent ion transport; gray arrows represent endocrine activity, gray-open arrows represent not-yet established endocrine activity; gray dotted arrows represent non-endocrine factors associated with the regulation or sensing activity. (1) Once the ovary is developed it (2) secrets the gonadal hormones; among them EST that together with other gonadal hormones induce oviduct development and maintenance, acts as anti apoptotic mean and stimulate (3) egg-white proteins synthesis. In addition, (4) EST induce liver formation of PBP and (5) induce (together with testosterone) medullary bone formation. (6) EST may have also a not-yetestablished specific effect on shell calcification. (7) PR affects specifically shell calcification and calbindin mRNA synthesis and appears to be a rational candidate for the “off” signal for shell calcification. (8) PG, formed in the follicles and (8a) in the oviduct, is believed to regulate (9) oviduct muscle contraction and egg oviposition (other hormones such as parathyroid hormone related peptide, either of autocrine or paracrine origin, or arginine vasotocin or oxytocin may also be involved in this activity). (10) The reduced plasma Ca2+ resulted from its binding to the PBP and/or its deposition in the medullary bone, and/or, to much greater extent, in the eggshell induces PTH secretion by the PTG. (11) PTH, as well as (12) the lower plasma (extracellular) Ca 2+ (13) induce the formation of renal 1,25-D3, whereas high extracellular Ca2+ reduces 1,25-D3 synthesis and induces renal calbindin synthesis. (14) 1,25-D3 induces intestinal Ca2+ absorption (see also Fig. 7) and (15) bone calcium mobilization. (16) 1,25D3 may have also an effect on ESG CA (not-yet-established) and (17) on the transfer of Ca 2+ into the shell gland lumen (most of the evidence reject this hypothesis). (18) 1,25-D3 down regulates its own synthesis by the kidney and (19) down regulates PTH formation by the PTG. (20) Both hormones (PTH and 1,25D3), as well as the extracellular Ca 2+, regulate renal reabsorption of Ca2+ and consequently urinary calcium. (21) PTH (and 1,25-D3; see also 15) affects bone remodeling (osteoclast activity). (22) Pressure sensitivity to mechanical strain resulted from egg white formation in the magnum and egg swelling (plumping) in the ESG (23) induces OPN mRNA formation in the isthmus and (24) the circadian synthesis of several other mRNAs in the ESG. (25) A Ca 2+-transport-related factor(s) induces ESG calbindin and few other ESG mRNAs synthesis. (26) H+ resulted fro ESG-CA induces acidosis and consequently calcium solubility in the gizzard and the intestinal lumen. 1352 The overall phenomenon is acceleration of Ca2+ flow from the gut lumen to the ESG lumen, 1353 where the eggshell is formed during the later phases of the egg cycle. Most likely, this flow is 1354 primarily driven by ESG-CA activity that results in high HCO3- content that, in turn, "sucks 1355 out" calcium from the intestinal lumen via the blood and ESG cells, and deposits it in the 1356 shell crystals. In the intestine, where the capacity to transport Ca2+ corresponds, in most cases, 1357 to the calbindin content (Bar et al. 1979a; Bar et al. 1979b), the transcellular pathway appears 1358 to provide an additional means to overcome low calcium intake, but the major driver remains 1359 the ECPD and its response to the variations in blood and intestinal lumen concentrations of 1360 Ca2+ during the egg cycle. Acidosis/H+ formation that result from CA activity during shell 1361 calcification may benefit the ECPD contribution to the paracellular Ca2+ transport, through 1362 their effect on calcium solubility in the gizzard and the intestinal lumen (see 3.2; 3.3.2.2; 1363 3.3.2.3; (Mongin 1976b; Mongin 1976a)). This may explain some of the discrepancy between 1364 calbindin content and the intestinal capability to absorb Ca+2 under certain conditions, such as 1365 onset of production (see 2.2; 3.1) or during shell calcification. It may also explain the specific 1366 effect of exogenous 1-hydroxylated active derivatives of vitamin D, which is observed almost 1367 exclusively during the period of shell calcification (see 2.2; 3.3.2). This supports the idea that 1368 intestinal paracellular Ca2+ transport is also dependent on vitamin D. The above-mentioned 1369 suggestion is further supported by the recent finding that calbindin-D9K is not required for 45 1370 vitamin D3-mediated Ca2+ absorption in the mammalian small intestine (Akhter et al. 2007; 1371 Benn et al. 2008). 1372 In the ESG, although TRPVs were not yet identified, the presence of the other two 1373 components (PMCA and calbindin) of transcellular transport Ca2+, the presence of TRPVs in 1374 the mammalian uterus and placenta, the localization of PMCA (see 3.2, 4.2, 4.4), and the 1375 apparently "uphill" transport of Ca2+ that occurs in the ESG, support the idea that Ca2+ is 1376 transported via the transcellular pathway. However, the lack of an effect of vitamin D on the 1377 ESG transport of Ca2+, the plasma-to-ESG lumen positive EPD, the lack of solid information 1378 on the Ca2+ concentrations in the luminal zone adjacent to the Ca2+-secreting cells, and the 1379 obvious and critical involvement of CA in this procedure, suggest that the mechanisms there 1380 could not be easily explained solely in terms of a simple mechanism of transcellular Ca2+ 1381 transport. If that were the case, the "active" Ca2+ transport would reflect the energy-dependent 1382 CA activity, which is also involved in shell deposition in the ESG, rather than true active Ca2+ 1383 transport. The Ca2+ transport takes place mostly, or at least to a great extent, via the 1384 paracellular routes, rather than via the energy-dependent transcellular Ca2+ transport. 1385 With regard to the paracellular transport, ESG calbindin, which is related to shell Ca2+ 1386 mass (Bar et al. 1984b; Nys et al. 1986b; Bar et al. 1988; Bar et al. 1992a; Bar et al. 1992b; 1387 Bar et al. 1999; Goto et al. 2002c), may provide buffering capacity that prevents the 1388 accumulation of excessive cellular Ca2+, or acts as an anti-apoptosis, rather than a 1389 transporting factor. 1390 The hypothesis that calbindin, a major component of the transcellular transport of 1391 calcium, has differing roles in Ca2+ transport in the intestine and in the ESG is strengthened 1392 by the differing roles played by vitamin D in calbindin synthesis by the two transporting 1393 organs (see 2.2). 1394 The overall mechanism and its hormonal control develop during the birds’ growth and 1395 during maturation, before the onset of egg laying, and then undergo accelerated development 1396 during the egg cycle. The early pre-laying regulation involves the growth and metabolism 1397 hormones, induction of the development of the functional ESG by the hypothalamus- 1398 pituitary-gonadal (neuroendocrine) hormones the formation of yolk- and plasma-calcium- 1399 binding proteins in the liver, and the establishment of the calcium reservoirs in the bones, 1400 especially the specific medullary bone. Because of the increased demands for Ca2+ for these 1401 reservoirs, the calcium-regulating hormones induce the development of intestinal absorptive 1402 capacity for calcium. The calcium-regulating and the gonadal hormones appear also to be 1403 involved in the development of the high ESG-CA capacity and, most likely, also in the 1404 development of ESG-PMCA and TPRVs (if occur there). However, the diurnal variation in 1405 calcium transport by the ESG is unlikely to be dependent on the calcium-regulating hormones 46 1406 (see 3.3). On the other hand, the plasma concentrations of many gonadal and neuroendocrine 1407 hormones fluctuate in close temporal association with shell formation during the egg cycle, 1408 and may be responsible for the variation in calcium transport or CA activity. Progesterone 1409 concentration peaked (Doi et al. 1980; Johnson et al. 1980; Nys et al. 1986a; Braw-Tal et al. 1410 2004) 2 to 6 h before termination of shell calcification, and a single i.m. injection of 1411 progesterone, but not of estrogen or testosterone, given while the egg was in the magnum, 1412 markedly inhibited shell calcification of the formed egg, delayed its oviposition (Nys 1987; 1413 Bar et al. 1996; Goto et al. 2002b), and inhibited calbindin gene transcription in the ESG but 1414 not in the intestine) (Bar et al. 1996; Goto et al. 2002b). Estrogen has only a tiny effect on 1415 ESG calbindin (See 2.2), therefore, the marked effect of progesterone could not be a result 1416 only of its anti-estrogenic (Bruns et al. 1988; Corradino 1993) activity. This suggests that 1417 progesterone may play a specific role in the "turn-off" signal that terminates shell 1418 calcification. Less evidence is available concerning the "turn on" signal for shell calcification: 1419 this signal, too, may be dependent, in an as yet undefined way, on the gonadal or 1420 neuroendocrine hormones, but it may also be initiated by the mechanical strain developed 1421 during "egg white" secretion in the magnum or during its swelling ("plumping") in the ESG 1422 just after the movement of the shell-less egg from the isthmus to the ESG (reviewed by 1423 (Solomon 1991; Etches 1996; Johnson 2000)). This hypothesis is supported by the findings 1424 that ESG Ca2+ secretion in situ during the period of shell calcification (predicated on the basis 1425 of the earlier ovulation) is further stimulated by the presence of an egg (or an egg-like insert) 1426 in the ESG (see 4.1). It was found that: an Na+ pump has a role in the ESG-CA activity (see 1427 also 2.5); that mechanical strain appears to regulate molecular events in the ESG, such as the 1428 expression of Na+-K+-ATPase (Lavelin et al. 2001) and of other genes (Lavelin et al. 1998; 1429 Lavelin et al. 2000; Lavelin et al. 2002); and mechanoreceptor candidates were already 1430 considered to be involved in another calcium-transporting tissue, i.e., the bone (reviewed in 1431 (Rubin et al. 2006) and others). In light of these findings, the possibility of a specific role of 1432 the mechanical strain that results from the formation of egg white in the magnum and 1433 "plumping" in the initiation of shell formation should not be ignored. Such a combined 1434 mechanism (pressure and the endocrine cycle) may be involved in the initiation of shell 1435 formation, via the completion of the OPN-containing mammillae on the egg outer membrane, 1436 where the seeding of the calcium crystals of the calcified shell is initiated. 1437 Although some aspects of the above speculation remain to be proved, such a hypothesis, 1438 that aims to account for the elevated transport of Ca2+ in the ESG and intestine during shell 1439 formation, should be considered seriously. 1440 47 1441 Acknowledgments 1442 Contribution from the Institute of Animal Science, Agricultural Research Organization, the 1443 Volcani Center, Bet Dagan, Israel: No. 532-08. 1444 The encouragement and comments of Prof. S. Yahav and the comments of Dr. Pines, ARO 1445 are acknowledged with appreciation. 1446 1447 1448 References 1449 1450 1451 1452 1453 1454 1455 1456 1457 1458 1459 1460 1461 1462 1463 1464 1465 1466 1467 1468 1469 1470 1471 1472 1473 1474 1475 1476 1477 1478 1479 1480 1481 1482 1483 1484 1485 1486 1487 Agemori, M., Ishikawa, K., 1984. Binding of progesterone with the oviduct cytosol fraction of estrogen-primed quail (Coturnix coturnix japonica). Gen. Comp. Endocrinol. 53, 17-27. Akhter, S., Kutuzova, G.D., Christakos, S., DeLuca, H.F., 2007. Calbindin D9k is not required for 1,25dihydroxyvitamin D3-mediated Ca2+ absorption in small intestine. Arch. Biochem. Biophys. 460, 227-232 Al Batshan, H.A., Scheideler, S.E., Black, B.L., Garlich, J.D., Anderson, K.E., 1994. Duodenal calcium uptake, femur ash, and eggshell quality decline with age and increase following molt. Poult. Sci. 73, 15901596. Anish, D., Sastry, K.V., Sundaresan, N.R., Saxena, V.K., Singh, R., Mohan, J., 2008. Reproductive tissue regression: involvement of caspases, inducible nitric oxide synthase and nitric oxide during moulting in White Leghorn hens. Anim. Reprod. Sci. 104, 329-343. Annaka, A., Watanabe, E., Sunahara, T., Ishibashi, T., 1989. Site and rate of absorption and secretion of calcium and phosphorus in laying hens. Bull .Fac. Agricult., Niigata University 41, 37-44. Arad, Z., Eylath, U., Ginsburg, M., Eyal Giladi, H., 1989. Changes in uterine fluid composition and acid-base status during shell formation in the chicken. Am. J. Physiol. 257, R732-R737. Arai, T., Sugiyama, T., Kusuhara, S., 1996. Immunohistochemical studies of Ca 2+-ATPase and carbonic anhydrase II in the shell gland of egg-laying hens. Jap. Poult. Sci. 33, 371-376. Arias, J.L., Fernandez, M.S., 2001. Role of extracellular matrix molecules in shell formation and structure. World's Poult. Sci. J. 57, 349-357. Armbrecht, H.J., Boltz, M.A., Wongsurawat, N., 1994. Expression of plasma membrane calcium pump mRNA in rat intestine: effect of age and 1,25-dihydroxyvitamin D. Biochim. Biophys. Acta 12, 110-114. Armbrecht, H.J., Zenser, T.V., Davis, B.B., 1980. Effect of vitamin D metabolites on intestinal calcium absorption and calcium-binding protein in young and adult rats. Endocrinology 106, 469-475. Armbrecht, H.J., Zenser, T.V., Davis, B.B., 1980. Effect of age on the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by kidney of rat. J. Clin. Invest. 66, 1118-1123. Balnave, D., 1993. Influence of saline drinking water on eggshell quality and formation. World's Poult. Sci. J. 49, 109-119. Balnave, D., El Khatib, N.U., Zhang, D., 1992. Calcium and carbonate supply in the shell gland of hens laying eggs with strong and weak shells and during and after a rest from lay. Poult. Sci. 71, 2035-2040. Bar, A., 2008. Calcium homeostasis, vitamin D metabolism and expression in strongly calcifying laying birds. Comp. Biochem, Physiol. A Mol Integr Physiol 151, 477-490. Bar, A., 2009. Calcium transport in strongly calcifying laying birds: Mechanisms and regulation. Comp Biochem Physiol A Mol Integr Physiol. in press. Bar, A., Cohen, A., Edelstein, S., Shemesh, M., Montecuccoli, G., Hurwitz, S., 1978a. Involvement of cholecalciferol metabolism in birds in the adaptation of calcium absorption to the needs during reproduction. Comp. Biochem, Physiol. B 59, 245-249. Bar, A., Cohen, A., Eisner, U., Risenfeld, G., Hurwitz, S., 1978b. Differential response of calcium transport systems in laying hens to exogenous and endogenous changes in vitamin D status. J. Nutr. 108, 13221328. 48 1488 1489 1490 1491 1492 1493 1494 1495 1496 1497 1498 1499 1500 1501 1502 1503 1504 1505 1506 1507 1508 1509 1510 1511 1512 1513 1514 1515 1516 1517 1518 1519 1520 1521 1522 1523 1524 1525 1526 1527 1528 1529 1530 1531 1532 1533 1534 1535 1536 1537 1538 1539 Bar, A., Dubrov, D., Eisner, U., Hurwitz, S., 1976a. Calcium-binding protein and calcium absorption in the laying quail (Coturnix coturnix japonica). Poult. Sci. 55, 622-628. Bar, A., Eisner, U., Montecuccoli, G., Hurwitz, S., 1976b. Regulation of intestinal calcium absorption in the laying quail: independent of kidney vitamin D hydroxylation. J. Nutr. 106, 1336-1342. Bar, A., Hurwitz, S., 1969a. In vitro calcium transport in laying fowl intestine: characterization of the system and medium composition. Poult. Sci. 48, 1105-1113. Bar, A., Hurwitz, S., 1969b. The accumulation of calcium in laying fowl intestine in vitro. Biochim. Biophys. Acta 183, 591-600. Bar, A., Hurwitz, S., 1972a. Relationship of duodenal calcium-binding protein to calcium absorption in the laying fowl. Comp. Biochem, Physiol. B 41, 735-744. Bar, A., Hurwitz, S., 1973a. Calcium restriction and intestinal calcium-binding protein in the laying fowl. Comp. Biochem, Physiol. A 45, 571-577. Bar, A., Hurwitz, S., 1973b. Uterine calcium-binding protein in the laying fowl. Comp. Biochem, Physiol. A 45, 579-586. Bar, A., Hurwitz, S., 1974. Bone ash, duodenal 45Ca absorption and calcium-binding protein in chicks fed lathyrogens. Comp. Biochem, Physiol. A 47, 1257-1263. Bar, A., Hurwitz, S., 1975a. Intestinal and uterine calcium-binding protein in laying hens during different stages of egg formation. Poult. Sci. 54, 1325-1327. Bar, A., Hurwitz, S., 1979a. The interaction between dietary calcium and gonadal hormones in their effect on plasma calcium, bone, 25-hydroxycholecalciferol-1-hydroxylase, and duodenal calcium-binding protein, measured by a radioimmunoassay in chicks. Endocrinology 104, 1455-1460. Bar, A., Hurwitz, S., 1981a. Relationships between cholecalciferol metabolism and growth in chicks as modified by age, breed and diet. J. Nutr. 111, 399-404. Bar, A., Hurwitz, S., 1984a. Egg shell quality, medullary bone ash, intestinal calcium and phosphorus absorption, and calcium-binding protein in phosphorus-deficient hens. Poult. Sci. 63, 1975-1979. Bar, A., Hurwitz, S., Cohen, I., 1972b. Relationship between duodenal calcium-binding protein, parathyroid activity and various parameters of mineral metabolism in the rachitic and vitamin D-treated chick. Comp. Biochem, Physiol. A 43, 519-526. Bar, A., Hurwitz, S., Edelstein, S., 1975b. Response of renal calcium-binding protein. Independence of kidney vitamin D hydroxylation. Biochim. Biophys. Acta 411, 106-112. Bar, A., Maoz, A., Hurwitz, S., 1979b. Relationship of intestinal and plasma calcium-binding protein to intestinal calcium absorption. FEBS Lett. 102, 79-81. Bar, A., Norman, A.W., 1981b. Studies on the mode of action of calciferol. XXXIV. Relationship of the distribution of 25-hydroxyvitamin D3 metabolites to gonadal activity and egg shell formation in the quail. Endocrinology 109, 950-955. Bar, A., Rosenberg, J., Hurwitz, S., 1984b. The lack of relationships between vitamin D 3 metabolites and calcium-binding protein in the eggshell gland of laying birds. Comp. Biochem, Physiol. B 78, 75-79. Bar, A., Shani, M., Fullmer, C.S., Brindak, M.E., Striem, S., 1990a. Modulation of chick intestinal and renal calbindin gene expression by dietary vitamin D3, 1,25-dihydroxyvitamin D3, calcium and phosphorus. Mol. Cell. Endocrinol. 72, 23-31. Bar, A., Shinder, D., Yosefi, S., Wax, E., Plavnik, I., 2003. Metabolism and requirements for calcium and phosphorus in the fast-growing chicken as affected by age. Br. J. Nutr. 89, 51-60. Bar, A., Striem, S., Mayel Afshar, S., Lawson, D.E., 1990b. Differential regulation of calbindin-D28K mRNA in the intestine and eggshell gland of the laying hen. J. Mol. Endocrinol. 4, 93-99. Bar, A., Striem, S., Rosenberg, J., Hurwitz, S., 1988. Egg shell quality and cholecalciferol metabolism in aged laying hens. J. Nutr. 118, 1018-1023. Bar, A., Striem, S., Vax, E., Talpaz, H., Hurwitz, S., 1992a. Regulation of calbindin mRNA and calbindin turnover in intestine and shell gland of the chicken. Am. J. Physiol. 262, R800-R805. Bar, A., Vax, E., Hunziker, W., Halevy, O., Striem, S., 1996. The role of gonadal hormones in gene expression of calbindin (Mr 28,000) in the laying hen. Gen. Comp. Endocrinol. 103, 115-122. Bar, A., Vax, E., Striem, S., 1992b. Relationships between calbindin (M r 28,000) and calcium transport by the eggshell gland. Comp. Biochem, Physiol. B 101, 845-848. 49 1540 1541 1542 1543 1544 1545 1546 1547 1548 1549 1550 1551 1552 1553 1554 1555 1556 1557 1558 1559 1560 1561 1562 1563 1564 1565 1566 1567 1568 1569 1570 1571 1572 1573 1574 1575 1576 1577 1578 1579 1580 1581 1582 1583 1584 1585 1586 1587 1588 1589 1590 Bar, A., Vax, E., Striem, S., 1998. Effects of age at onset of production, light regime and dietary calcium on performance, eggshell traits, duodenal calbindin and cholecalciferol metabolism. Br. Poult. Sci.. 39, 282-290. Bar, A., Vax, E., Striem, S., 1999. Relationships among age, eggshell thickness and vitamin D metabolism and its expression in the laying hen. Comp. Biochem, Physiol. A 123, 147-154. Bar, A., Wasserman, R.H., 1973c. Control of calcium absorption and intestinal calcium-binding protein synthesis. Biochem. Biophys. Res. Commun. 54, 191-196. Belkacemi, L., Bedard, I., Simoneau, L., Lafond, J., 2005. Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium 37, 1-8. Benesch, R., Barron, N.S., Mawson, C.A., 1944. Carbonic anhydrase, sulphonamides and shell formation in the domestic fowl. Nature 153, 138-139. Benn, B.S., Ajibade, D., Porta, A., Dhawan, P., Hediger, M., Peng, J.B., Jiang, Y., Oh, G.T., Jeung, E.B., Lieben, L., Bouillon, R., Carmeliet, G., Christakos, S., 2008. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 149, 31963205. Benoit, J., Clavert, J., 1944. Hypercalcemia et osteogens medullaire foilliculiniques chez le canard domestique soumis a un regime a calcique. Cr. Soc. Biol. Paris 139, 737-742. Bernstein, R.S., Nevalainen, T., Schraer, R., Schraer, H., 1968. Intracellular distribution and role of carbonic anhydrase in the avian shell gland mucosa. Biochem. Biophys. Acta 159, 367-376. Bielorai, R., Tamir, M., Alumot, E., Bar, A., Hurwitz, S., 1973. Digestion and absorption of protein along the intestinal tract of chicks fed raw and heated soybean meal. J. Nutr. 103, 1291-1298. Billecocq, A., Emanuel, J.R., Levenson, R., Baron, R., 1990. 1 alpha 25-dihydroxyvitamin D3 regulates the expression of carbonic anhydrase II in nonerythroid avian bone marrow cells Proc. Natl. Acad. Sci. U S A 87, 6470-6474. Bitman, J., Cecil, H.C., Fries, G.F., 1970. DDT-induced inhibition of avian shell gland carbonic anhydrase: a mechanism for thin eggshells. Science 168, 594-596. Borke, J.L., Caride, A., Verma, A.K., Kelley, L.K., Smith, C.H., Penniston, J.T., Kumar, R., 1989a. Calcium pump epitopes in placental trophoblast basal plasma membranes. Am. J. Physiol. 257, C341-C346. Borke, J.L., Caride, A., Verma, A.K., Penniston, J.T., Kumar, R., 1989b. Plasma membrane calcium pump and 28-kDa calcium binding protein in cells of rat kidney distal tubules. Am. J. Physiol. 257, F842-849. Borke, J.L., Caride, A., Verma, A.K., Penniston, J.T., Kumar, R., 1990. Cellular and segmental distribution of Ca2(+)-pump epitopes in rat intestine. Pflugers Arch 417, 120-122. Bouillon, R., Carmeliet, G., Boonen, S., 1997. Ageing and calcium metabolism. Bailliere Clin Endocrinol Met 11, 341-365. Bouillon, R., Van Cromphaut, S., Carmeliet, G., 2003. Intestinal calcium absorption: Molecular vitamin D mediated mechanisms. J. Cell. Biochem. 88, 332-339. Braw-Tal, R., Yossefi, S., Pen, S., Shinder, D., Bar, A., 2004. Hormonal changes associated with ageing and induced moulting of domestic hens. Br. Poult. Sci.. 45, 815-822. Bronner, F., 2003. Mechanisms of intestinal calcium absorption. J. Cell. Biochem. 88, 387-393. Brown, A.J., Dusso, A., Slatopolsky, E., 1999. Vitamin D. Am. J. Physiol. 46, F157-F175. Brown, A.J., Krits, I., Armbrecht, H.J., 2005. Effect of age, vitamin D, and calcium on the regulation of rat intestinal epithelial calcium channels. Arch. Biochem. Biophys. 437, 515-518. Brown, D., Roth, J., Kumpulainen, T., Orci, L., 1982. Ultrastructural immunocytochemical localization of carbonic anhydrase. Presence in intercalated cells of the rat collecting tubule. Histochemistry 75, 209213. Bruns, M.E., Overpeck, J.G., Smith, G.C., Hirsch, G.N., Mills, S.E., Bruns, D.E., 1988. Vitamin D-dependent calcium binding protein in rat uterus: differential effects of estrogen, tamoxifen, progesterone, and pregnancy on accumulation and cellular localization. Endocrinology 122, 2371-2378. Butler, W.T., 1989. The nature and significance of osteopontin. Connect Tissue Res 23, 123-136. Butler, W.T., Ridall, A.L., McKee, M.D., 1996. Osteopontin. In: Bilezikian, J.P., Raisz, L.C., Rodan, G.A. (Eds.), Principles of Bone Biology, Academic Press, San Diego, pp. 167-181 50 1591 1592 1593 1594 1595 1596 1597 1598 1599 1600 1601 1602 1603 1604 1605 1606 1607 1608 1609 1610 1611 1612 1613 1614 1615 1616 1617 1618 1619 1620 1621 1622 1623 1624 1625 1626 1627 1628 1629 1630 1631 1632 1633 1634 1635 1636 1637 1638 1639 1640 1641 1642 1643 Cai, Q., Chandler, J.S., Wasserman, R.H., Kumar, R., Penniston, J.T., 1993. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proc. Natl. Acad. Sci. U S A 90, 1345-1349. Centeno, V.A., Diaz-de-Barboza, G.E., Marchionatti, A.M., Alisio, A.E., Dallorso, M.E., Nasif, R., Tolosa-deTalamoni, N.G., 2004. Dietary calcium deficiency increases Ca2+ uptake and Ca2+ extrusion mechanisms in chick enterocytes. Comp. Biochem. Physiol. A, Molecular and Integrative Physiology 139, 133-141. Chandra, S., Fullmer, C., Smith, C.A., Wasserman, R.H., Morrison, G.H., 1990. Ion microscopic imaging of calcium transport in the intestinal tissue of vitamin D-deficient and vitamin D-replete chickens: a 44Ca stable isotope study. Proc. Natl. Acad. Sci. USA 87, 5715-5719. Chandramoni, Jadhao, S.B., Sinha, R.P., 1998. Effect of dietary calcium and phosphorus concentrations on retention of these nutrients by caged layers. Brit. Poult. Sci. 39, 544-548. Charoenphandhu, N., Tudpor, K., Pulsook, N., Krishnamra, N., 2006. Chronic metabolic acidosis stimulated transcellular and solvent drag-induced calcium transport in the duodenum of female rats. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G446-455. Chien, Y.C., Hincke, M.T., Vali, H., McKee, M.D., 2008. Ultrastructural matrix-mineral relationships in avian eggshell, and effects of osteopontin on calcite growth in vitro. J. Struct. Biol. 163, 84-99. Chien, Y.C., Hincke, M.T., McKee, M.D., 2009. Avian Eggshell Structure and Osteopontin. Cells Tissues Organs. 189,38-43. Choi, K.C., Leung, P.C., Jeung, E.B., 2005. Biology and physiology of Calbindin-D9k in female reproductive tissues: involvement of steroids and endocrine disruptors. Reprod. Biol. Endocrinol. 3, 66. Christakos, S., Barletta, F., Huening, M., Dhawan, P., Liu, Y., Porta, A., Peng, X., 2003. Vitamin D target proteins: function and regulation. J. Cell. Biochem. 88, 238-244. Christakos, S., Liu, Y., Dhawan, P., Peng, X., 2005. The calbindins: calbindin-D9K and calbindin-D28K. In: Feldman, D., Pike, W.J., Glorieux, F.H. (Eds.), Vitamin D, Elsevier Academic Press, Burlington, MA; San Diego, CA; London, UK, pp. 721-735. Christakos, S., Dhawan, P., Benn, B., Porta, A., Hediger, M., Oh, G.T., Jeung, E.B., Zhong, Y., Ajibade, D., Dhawan, K., Joshi, S., 2007. Vitamin D: molecular mechanism of action. Ann. N. Y. Acad. Sci. 1116, 340-348. Clemens, T.L., McGlade, S.A., Garrett, K.P., Craviso, G.L., Hendy, G.N., 1989. Extracellular calcium modulates vitamin D-dependent calbindin-D28K gene expression in chick kidney cells. Endocrinology 124, 1582-1584. Clunies, M., Etches, R.J., Fair, C., Leeson, S., 1993. Blood, intestinal and skeletal calcium dynamics during egg formation. Canad. J. Anim. Sci.73, 517-532. Cohen, A., Bar, A., Eisner, U., Hurwitz, S., 1978. Calcium absorption, calcium-binding protein, and egg shell quality in laying hens fed hydroxylated vitamin D derivatives. Poult. Sci. 57, 1646-1651. Cohen, I., Hurwitz, S., 1973. The electrical potential difference and the short circuit current of the uterine mucosa of hens in relation to egg shell formation. Poult. Sci. 52, 2340-2341. Cohen, I., Hurwitz, S., 1974. Intracellular pH and electrolyte concentration in the uterine wall of the fowl in relation to shell formation and dietary minerals. Comp. Biochem, Physiol. A 49, 689-696. Comar, C.L., Driggers, J.C., 1949. Secretion of radioactive calcium in the hen's egg. Science 109, 282. Corradino, R.A., 1973. Embryonic chick intestine in organ culture. A unique system for the study of the intestinal calcium absorptive mechanism. J. Cell. Biol. 58, 64-78. Corradino, R.A., 1993. Calbindin D28K regulation in precociously matured chick egg shell gland in vitro. Gen. Comp. Endocrinol. 91, 158-166. Corradino, R.A., Alaimo, J., 1995. In vitro regulation of calbindinD28K mRNA by 1,25-dihydroxyvitamin D3 and estradiol in precociously-matured egg shell gland from vitamin D3-depleted chicks. Horm. Metab. Res.27, 39-40. Corradino, R.A., Fullmer, C.S., 1991. Positive cotranscriptional regulation of intestinal calbindin-D28K gene expression by 1,25-dihydroxyvitamin- D3 and glucocorticoids. Endocrinology 128, 944-950. Corradino, R.A., Smith, C.A., Krook, L.P., Fullmer, C.S., 1993. Tissue-specific regulation of shell gland calbindin D28K biosynthesis by estradiol in precociously matured, vitamin D-depleted chicks. Endocrinology 132, 193-198. 51 1644 1645 1646 1647 1648 1649 1650 1651 1652 1653 1654 1655 1656 1657 1658 1659 1660 1661 1662 1663 1664 1665 1666 1667 1668 1669 1670 1671 1672 1673 1674 1675 1676 1677 1678 1679 1680 1681 1682 1683 1684 1685 1686 1687 1688 1689 1690 1691 1692 1693 Corradino, R.A., Wasserman, R.H., Pubols, M.H., Chang, S.I., 1968. Vitamin D 3 induction of a calcium-binding protein in the uterus of the laying hen. Arch. Biochem. Biophys. 125, 378-380. Coty, W.A., 1980. A specific, high affinity binding protein for 1,25-dihydroxyvitamin D in the chick oviduct shell gland. Biochem. Biophys. Res. Commun. 93, 285-292. Cramer, C.F., 1965. Sites of calcium absorption and the calcium concentration of gut contents in the dog. Can. J. Physiol. Pharmacol. 43, 75-78. Cramer, C.F., Dueck, J., 1962. In vivo transport of calcium from healed Thiry-Vella fistulas in dogs. Am. J. Physiol. 202, 161-164. Criddle, R.A., Zheng, M.H., Dick, I.M., Callus, B., Prince, R.L., 1997. Estrogen responsiveness of renal Calbindin-D-28k gene expression in rat kidney. J. Cell. Biochem. 65, 340-348. Dacke, C.G., 1976. Parathyroid hormone and eggshell calcification in Japanese quail. J. Endocrinol. 71, 239243. Dacke, C.G., 1979. Calcium Regulation in Sub-Mammalian Verterbrates. Acadim Press, London, NY, SF. Dacke, C.G., 2000. The parathyroids, calcitonin, and vitamin D. In: Whittow, G.C. (Ed.) Sturkie’s Avian Physiology, Academic Press, New York,, pp. 473-488 Dacke, C.G., Musacchia, X.J., Volkert, W.A., Kenny, A.D., 1973. Cyclical fluctuations in the levels of blood calcium, pH and pCO 2 in Japanese quail. Comp. Biochem, Physiol. A 44, 1267-1275. Davis, R.A., 1997. Evolution of processes and regulators of lipoprotein synthesis: from birds to mammals. J. Nutr. 127, 795S-800S. Davis, W.L., Jones, R.G., Farmer, G.R., Matthews, J.L., Martin, J.H., Bridges, G., 1987. Electron microscopic cytochemical localization of a basolateral calcium adenosine triphosphatase in vitamin D replete chick enterocytes. Anat. Rec. 219, 384-393. DeLuca, H.F., 2004. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 80, 1689S-1696S. Dick, I.M., Liu, J., Glendenning, P., Prince, R.L., 2003. Estrogen and androgen regulation of plasma membrane calcium pump activity in immortalized distal tubule kidney cells. Mol. Cell. Endocrinol. 212, 11-18. Doi, O., Takai, T., Nakamura, T., Tanabe, Y., 1980. Changes in the pituitary and plasma LH, plasma and follicular progesterone and estradiol, and plasma testosterone and estrone concentrations during the ovulatory cycle of the quail (Coturnix coturnix japonica). Gen. Comp. Endocrinol. 41, 156-163. Driggers, J.C., Comar, C.L., 1949. The secretion of radioactive calcium (45Ca) in the hen’s egg. Poult. Sci. 28, 420-424. Dulce, H.J., Siegmund, P., Koerber, F., Schuette, E., 1960. [On the biochemistry of the dissolution of bone. 3. On the presence of carbonic anhydrase in bones. Hoppe-Seylers Z. Physiol. Chem. 320, 163-167. Dusso, A.S., Brown, A.J., Slatopolsky, E., 2005. Vitamin D. Am. J. Physiol. Renal Physiol 289, F8-28. Eastin, W.C., Jr., Spaziani, E., 1978a. On the control of calcium secretion in the avian shell gland (uterus). Biol. Reprod. 19, 493-504. Eastin, W.C., Jr., Spaziani, E., 1978b. On the mechanism of calcium secretion in the avian shell gland (uterus). Biol. Reprod. 19, 505-518. Edwards, H.M., 2000. Nutrition and skeletal problems in poultry. Poult. Sci. 79, 1018-1023. Edwards, N.A., 1977. The secretion of glucose into the egg of Gallus domesticus and observations on the uterine secretion of cations. Br. Poult. Sci. 18, 641-649. Ehrenspeck, G., Schraer, H., Schraer, R., 1967. Some metabolic aspects of calcium movement across the isolated avian shell gland. Proc. Soc. Exp. Biol. Med. 126, 392-395. Ehrenspeck, G., Schraer, H., Schraer, R., 1971. Calcium transfer across isolated avian shell gland. Am. J. Physiol. 220, 967-972. el Jack, M.H., Lake, P.E., 1967. The content of the principal inorganic ions and carbon dioxide in uterine fluids of the domestic hen J. Reprod. Fertil. 13, 127-132. Elbrond, V.S., Jones, C.J., Skadhauge, E., 2004. Localization, morphology and function of the mitochondria-rich cells in relation to transepithelial Na +-transport in chicken lower intestine (coprodeum). Comp. Biochem, Physiol. A Mol. Integr. Physiol. 137, 683-696. 52 1694 1695 1696 1697 1698 1699 1700 1701 1702 1703 1704 1705 1706 1707 1708 1709 1710 1711 1712 1713 1714 1715 1716 1717 1718 1719 1720 1721 1722 1723 1724 1725 1726 1727 1728 1729 1730 1731 1732 1733 1734 1735 1736 1737 1738 1739 1740 1741 1742 1743 1744 Esbaugh, A.J., Tufts, B.L., 2006. The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respir. Physiol. Neurobiol. 154, 185-198. Etches, R.J., 1990. The ovulatory cycle of the hen. CRC Crit. Rev. Poult. Biol. 2, 293-318. Etches, R.J., 1996. Reproduction in Poultry. CAP International, Cambridge. Favus, M.J., Bushinsky, D.A., Coe, F.L., 1986. Effects of medium pH on duodenal and ileal calcium active transport in the rat. Am. J. Physiol. 251, G695-700. Feher, J.J., 1984. Measurement of facilitated calcium diffusion by soluble calcium-binding protein. Biochem. Biophys. Acta. 773, 91-98. Feher, J.J., Fullmer, C.S., Wasserman, R.H., 1992. Role of facilitated diffusion of calcium by calbindin in intestinal calcium absorption. Am. J. Physiol. 262, C517-C526. Fernandez, M.S., Escobar, C., Lavelin, I., Pines, M., Arias, J.L., 2003. Localization of osteopontin in oviduct tissue and eggshell during different stages of the avian egg laying cycle. J. Struct. Biol. 143, 171-180. Fernandez, M.S., Passalacqua, K., Arias, J.I., Arias, J.L., 2004. Partial biomimetic reconstitution of avian eggshell formation. J. Struct. Biol. 148, 1-10. Fox, J., Heath, H., 1981. Retarded growth rate caused by glucocorticoid treatment or dietary restriction: associated changes in duodenal, jejunal, and ileal calcium absorption in the chick. Endocrinology 108, 1138-1141. Freund, T., Bronner, F., 1975. Regulation of intestinal calcium-binding protein calcium intake in the rat. Am. J. Physiol. 228, 861-869. Friedlander, E.J., Henry, H.L., Norman, A.W., 1977. Studies on the mode of action of calciferol. Effects of dietary calcium and phosphorus on the relationship between the 25-hydroxyvitamin D3-1alphahydroxylase and production of chick intestinal calcium binding protein. J. Biol. Chem. 252, 86778683. Frost, T.J., Roland, D.A., Untawale, G.G., 1990. Influence of vitamin D 3, 1 alpha-hydroxyvitamin D3, and 1,25dihydroxyvitamin D3 on eggshell quality, tibia strength, and various production parameters in commercial laying hens. Poult. Sci. 69, 2008-2016. Fujita, H., Sugimoto, K., Inatomi, S., Maeda, T., Osanai, M., Uchiyama, Y., Yamamoto, Y., Wada, T., Kojima, T., Yokozaki, H., Yamashita, T., Kato, S., Sawada, N., Chiba, H., 2008. tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 19, 1912-1921. Fullmer, C.S., Brindak, M.E., Bar, A., Wasserman, R.H., 1976. The purification of calcium binding protein from the uterus of the laying hen. Proc. Soc. Exp. 152, 237-241. Fullmer, C.S., Chandra, S., Smith, C.A., Morrison, G.H., Wasserman, R.H., 1996. Ion microscopic imaging of calcium during 1,25-dihydroxyvitamin D-mediated intestinal absorption. Histochemistry Cell Biol. 106, 215-222. Fullmer, C.S., Wasserman, R.H., 1987. Chicken intestinal 28-kilodalton calbindin-D: complete amino acid sequence and structural considerations. Proc. Natl. Acad. Sci. U S A 84, 4772-4776. Furr, B.J.A., Bonney, R.C., England, R.J., Cunningham, F.J., 1973. Luteinizing hormone and progesterone in peripheral blood during the ovulatory cycle of the hen, Gallus domesticus. J. Endocrinol. 57, 159-169. Gabriella, M.G., Menghi, G., 1994. The integrative segment of the quail Coturnix coturnix japonica. Occurrence and distribution of carbonic anhydrase and complex carbohydrates. J Anat 185 ( Pt 2), 405-614. Gabrielli, M.G., Vincenzetti, S., Vita, A., Menghi, G., 1998. Immunohistochemical localization of carbonic anhydrase isoenzymes II and III in quail kidney. Histochem. J.30, 489-497. Gafter, U., Kraut, J.A., Lee, D.B., Silis, V., Walling, M.W., Kurokawa, K., Haussler, M.R., Coburn, J.W., 1980. Effect of metabolic acidosis in intestinal absorption of calcium and phosphorus. Am. J. Physiol. 239, G480-484. Gay, C.V., Mueller, W.J., 1974. Carbonic anhydrase and osteoclasts: localization by labeled inhibitor autoradiography. Science 183, 432-434. Ghijsen, W.E., De Jong, M.D., Van Os, C.H., 1983. Kinetic properties of Na +/Ca2+ exchange in basolateral plasma membranes of rat small intestine. Biochim. Biophys. Acta 730, 85-94. Gilbert, A.B., 1983. Calcium and reproductive function in the hen. Proc. Nutr. Soc. 42, 195-212. 53 1745 1746 1747 1748 1749 1750 1751 1752 1753 1754 1755 1756 1757 1758 1759 1760 1761 1762 1763 1764 1765 1766 1767 1768 1769 1770 1771 1772 1773 1774 1775 1776 1777 1778 1779 1780 1781 1782 1783 1784 1785 1786 Gill, R.K., Christakos, S., 1995. Regulation by estrogen through the 5'-flanking region of the mouse calbindinD28K gene. Mol. Endocrinol. 9, 319-326. 1787 1788 1789 1790 1791 1792 1793 1794 1795 Henry, H.L., 2005. The 25-hydroxyvitamin D 1 -hydroxylase. In: Feldman, D., Pike, W.J., Glorieux, F.H. (Eds.), Vitamin D, Elsevier Academic Press, Burlington, MA; San Diego, CA; London, UK, pp. 5768. Gill, S.P., Gangwar, P.C., 1990. Carbonic anhydrase and its isozymes in heat stressed white Leghorn hens. Arch. Exp. Veterinarmed. 44, 383-388. Glendenning, P., Ratajczak, T., Prince, R.L., Garamszegi, N., Strehler, E.E., 2000. The promoter region of the human PMCA1 gene mediates transcriptional downregulation by 1,25-dihydroxyvitamin D3. Biochem. Biophys. Res. Commun. 277, 722-728. Goto, H., Tagami, M., Aruga, M., Takama, T., Takeishi, M., Yamamura, N., Kamiyoshi, M., 2002a. Influence of time of calcium intake on plasma concentrations of calcium and shell gland concentration of CaBPD28KmRNA. Jap. Poult. Sci. 39, J1-J10. Goto, H., Tagami, M., Ina, S., Arakawa, N., Yamamura, N., Takeishi, M., Kamiyoshi, M., 2002b. Effect of sex steroid hormone on gene expression of calcium-binding protein (CaBP-D28K) in the shell gland. Jap. Poult. Sci. 39, J11-J24. Goto, H., Tagami, M., Mizuno, M., Kameoka, E., Takeishi, M., Yamamura, N., Doi, O., Kamiyoshi, M., 2002c. Plasma calcium concentrations and expression of CaBP-D28K mRNA in the shell gland in relation to calcification in the laying hen. Jap. Poult. Sci. 39, J25-J40. Goulding, A., Campbell, D.R., 1984. Thyroparathyroidectomy exaggerates calciuric action of ammonium chloride in rats. Am. J. Physiol. 246, F54-58. Griffin, H.D., 1992. Manipulation of egg yolk cholesterol–a physiologist's view. World Poult. Sci. J. 48, 101112. Gross, M., Kumar, R., 1990. Physiology and biochemistry of vitamin D-dependent calcium binding proteins. Am. J. Physiol. 259, F195-209. Grunder, A.A., Hollands, K.G., 1976. Carbonic anhydrase isozymes of blood and oviducal tissues of Leghorn hens. Poult. Sci. 55, 2255-2261. Grunder, A.A., Tsang, C.P., Narbaitz, R., 1990. Effects of vitamin D 3 metabolites on physiological traits of White Leghorn. Poult. Sci. 69, 1204-1208. Guinotte, F., Nys, Y., Monredon, F.d., 1991. The effects of particle size and origin of calcium carbonate on performance and ossification characteristics in broiler chicks. Poultry science 70, 1908-1920. Gulati, D.P., Nakamura, T., Tanabe, Y., 1981. Diurnal variations in plasma LH, progesterone, testosterone, estradiol, and estrone in the Japanese quail. Poult. Sci. 60, 668-673. Han, K.H., Jung, J.Y., Cha, J.H., Kim, H., Madsen, K.M., Kim, J., 2003. 1,25-Dihydroxyvitamin D3 stimulates osteopontin expression in rat kidney. Nephron physiol. 93, 76-86. Harrison, H.E., Harrison, H.C., 1960. Transfer of Ca 45 across intestinal wall in vitro in relationship to action of vitamin D and cortisol. Am. J. Physiol. 199, 265-271. Haussler, M.R., Whitfield, G.K., Haussler, C.A., Hsieh, J.C., Thompson, P.D., Selznick, S.H., Dominguez, C.E., Jurutka, P.W., 1998. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J. Bone. Miner. Res. 13, 325-349. Haynes, N.B., Cooper, K.J., Kay, M.J., 1973. Plasma progesterone concentration in the hen in relation to the ovulatory cycle. Brit. Poult. Sci. 14, 349-357. Heizmann, C.W., Hunziker, W., 1990. Intracellular Calcium-Binding Molecules. In: Bronner, F. (Ed.) Intracellular Calcium Regulation, Alan R. Liss, Inc, New York, pp. 211-248 Hemmingsen, C., 2000. Regulation of renal calbindin-D28K. Pharmacol Toxicol 87 Suppl 3, 5-30. Heryanto, B., Yoshimura, Y., Tamura, T., 1997. Cell proliferation in the process of oviducal tissue remodeling during induced molting in hens. Poult. Sci. 76, 1580-1586. Hoenderop, J.G., Bindels, R.J., 2005a. Epithelial Ca2+ and Mg2+ channels in health and disease. J. Am. Soc. Nephro.l 16, 15-26. Hoenderop, J.G., Nilius, B., Bindels, R.J., 2005b. Calcium absorption across epithelia. Physiol. Rev. 85, 373422. 54 1796 1797 1798 1799 1800 1801 1802 1803 1804 1805 1806 1807 1808 1809 1810 1811 1812 1813 1814 1815 1816 1817 1818 1819 1820 1821 1822 1823 1824 1825 1826 1827 1828 1829 1830 1831 1832 1833 1834 1835 1836 1837 1838 1839 1840 1841 1842 1843 1844 1845 Holdsworth, E.S., Jordan, J.E., Keenan, E., 1975. Effects of cholecalciferol on the translocation of calcium by non-everted chick ileum in vitro. Biochem. J. 152, 181-190. Holick, M.F., 2003. Vitamin D: A millenium perspective. J. Cell. Biochem. 88, 296-307. Holm, L., Berg, C., Brunstrom, B., Ridderstrale, Y., Brandt, I., 2001. Disrupted carbonic anhydrase distribution in the avian shell gland following in ovo exposure to estrogen. Arch. Toxicol.75, 362-368. Holm, L., Blomqvist, A., Brandt, I., Brunstrom, B., Ridderstrale, Y., Berg, C., 2006. Embryonic exposure to o,p'-DDT causes eggshell thinning and altered shell gland carbonic anhydrase expression in the domestic hen. Environ. Toxicol. Chem. 25, 2787-2793. Holm, L., Ruziwa, S.D., Dantzer, V., Ridderstraale, Y., 2000. Carbonic anhydrase in the utero-vaginal junction of immature and mature ostriches. Brit. Poult. Sci. 41, 244-249. Holmes, R.S., 1977. Purification, molecular properties and ontogeny of carbonic anhydrase isozymes. Evidence for A, B and C isozymes in avian and mammalian tissues. Eur. J. Biochem. 78, 511-520. Hora, J., Gosse, B., Rasmussen, K., Spelsberg, T.C., 1986. Estrogen regulation of the biological activity of the avian oviduct progesterone receptor and its ability to induce avidin. Endocrinology 119, 1118-1125. Howard, A., Legon, S., Walters, J.R., 1993. Human and rat intestinal plasma membrane calcium pump isoforms. Am. J. Physiol. 265, G917-G925. Huang, C.L., Sun, L., Moonga, B.S., Zaidi, M., 2006. Molecular physiology and pharmacology of calcitonin. Cell Mol. Biol. (Noisy-le-grand) 52, 33-43. Hansard, S.L., Crowder, H.M., 1957. The physiological behavior of calcium in the rat. J. Nutr. 62, 325-339. Hunziker, W., 1986. The 28-kDa vitamin D-dependent calcium-binding protein has a six domain structure. Proc. Natl. Acad. Sci. USA 83, 7578-7582. Hurwitz, S., 1964. Bone composition and Ca45 retention in fowl as influenced by egg formation. Am. J. Physiol. 206, 198-204. Hurwitz, S., 1990. Calcium homeostasis in avian species. Prog. Clin. Biol. Res. 342, 586-591. Hurwitz, S., 1992. The role of vitamin D in poultry bone biology. In: Whithead, C.C. (Ed.) Bone Biology and Skeletal Disorders in Poultry, vol. 23. Carfax, Abingdon, pp. 87-102 Hurwitz, S., 1996. Homeostatic control of plasma calcium concentration. Crit Rev Biochem Mol Biol 31, 41100. Hurwitz, S., Bar, A., 1965. Absorption of calcium and phosphorus along the gastrointestinal tract of the laying fowl as influenced by dietary calcium and egg shell formation. J. Nutr. 86, 433-438. Hurwitz, S., Bar, A., 1966a. Calcium depletion and repletion in laying hens. I. Effect on calcium in various bone segments, in egg shells and in blood plasma, and on calcium balance. Poult. Sci. 45, 345-352. Hurwitz, S., Bar, A., 1966b. Rate of passage of calcium-45 and yttrium-91 along the intestine, and calcium absorption in the laying fowl. J. Nutr. 89, 311-316. Hurwitz, S., Bar, A., 1967a. Calcium metabolism of hens secreting heavy or light egg shells. Poult. Sci. 46, 1522-1527. Hurwitz, S., Bar, A., 1968. Activity, concentration, and lumen-blood electrochemical potential difference of calcium in the intestine of the laying hen. J. Nutr. 95, 647-654. Hurwitz, S., Bar, A., 1969a. Intestinal calcium absorption in the laying fowl and its importance in calcium homeostasis. Am. J. Clin. Nutr. 22, 391-395. Hurwitz, S., Bar, A., 1969b. Relation between the lumen-blood electrochemical potential difference of calcium, calcium absorption and calcium-binding protein in the intestine of the fowl. J. Nutr. 99, 217-224. Hurwitz, S., Bar, A., 1971. Calcium and phosphorus interrelationships in the intestine of the fowl. J. Nutr. 101, 677-685. Hurwitz, S., Bar, A., 1972. Site of vitamin D action in chick intestine. Am. J. Physiol. 222, 761-767. Hurwitz, S., Bar, A., 1996. Response of the calcium-regulating system in chickens to growth. In: Dacke, C., Danks, J., Caple, I., Flik, G. (Eds.), The comparative endocrinology of calcium regulation, Bourn Press, England, pp. 137-142. Hurwitz, S., Bar, A., Cohen, I., 1973a. Regulation of calcium absorption by fowl intestine. Am. J. Physiol. 225, 150-154. 55 1846 1847 1848 1849 1850 1851 1852 1853 1854 1855 1856 1857 1858 1859 1860 1861 1862 1863 1864 1865 1866 1867 1868 1869 1870 1871 1872 1873 1874 1875 1876 1877 1878 1879 1880 1881 1882 1883 1884 1885 1886 1887 1888 1889 1890 1891 1892 1893 1894 1895 1896 1897 Hurwitz, S., Bar, A., Katz, M., Sklan, D., Budowski, P., 1973b. Absorption and secretion of fatty acids and bile acids in the intestine of the laying fowl. J. Nutr. 103, 543-547. Hurwitz, S., Cohen, I., Bar, A., 1970. The transmembrane electrical potential difference in the uterus (shell gland) of birds. [Chickens, quail]. Comp. Biochem, Physiol. A 35, 873-878. Hurwitz, S., Griminger, P., 1960. Observations on the calcium balance of laying hens. J. Agric. Sci.189, 373377. Hurwitz, S., Griminger, P., 1961. Partition of calcium and phosphorus excretion in the laying hen. Nuture 189, 759-760. Hurwitz, S., Griminger, P., 1962. Estimation of calcium and phosphorus requirement in laying hen by balance technique. J. Sci Food Agric 13, 185-191. Hurwitz, S., Harrison, H.C., Harrison, H.E., 1967b. Effect of vitamin D 3 on the in vitro transport of calcium by the chick intestine. J. Nutr. 91, 319-323. Hurwitz, S., Plavnik, I., Shapiro, A., Wax, E., Talpaz, H., Bar, A., 1995. Calcium metabolism and requirements of chickens are affected by growth. J. Nutr. 125, 2679-2686. Ieda, T., Saito, N., Ono, T., Shimada, K., 1995. Effects of presence of an egg and calcium deposition in the shell gland on levels of messenger ribonucleic acid of CaBP-D-28K and of vitamin D-3 receptor in the shell gland of the laying hen. Gen. Comp. Endocrinol. 99, 145-151. Ieda, T., Saito, N., Shimada, K., 1999. Effect of low calcium diet on messenger ribonucleic acid levels of calbindin-D28K of intestine and shell gland in laying hens in relation to egg shell quality. Japan Poult. Sci. 36, 295-303. Ingersoll, R.J., Wasserman, R.H., 1971. Vitamin D 3-induced calcium-binding protein. Binding characteristics, conformational effects, and other properties. J. Biol. Chem. 246, 2808-2814. Ingleton, P.M., 2002. Parathyroid hormone-related protein in lower vertebrates. Comp. Biochem, Physiol. B 132B, 87-95. Jande, S.S., Tolnai, S., Lawson, D.E., 1981. Immunohistochemical localization of vitamin D-dependent calciumbinding protein in duodenum, kidney, uterus and cerebellum of chickens. Histochemistry 71, 99-116. Johnson, A.I., van Tienhoven, A., 1980. Plasma concentrations of six steroids and LH during the ovulatory cycle of the hen, Gallus domesticus. Biol. Reprod. 23, 386-393. Johnson, A.L., 2000. Reproduction in the female. In: Whittow, G.C. (Ed.) Sturkie's Avian Physiology, Academic Press, San Diego, pp. 569-596 Jones, G., Strugnell, S.A., DeLuca, H.F., 1998. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 78, 1193-1231. Kaetzel, D.M., Jr., Soares, J.H., Jr., 1985. Effect of dietary calcium stress on plasma vitamin D 3 metabolites in the egg-laying Japanese quail. Poult. Sci. 64, 1121-1127. Kamar, G.A.R., Hassan, H.A.M., Kicka, M.A., Stino, F.K.R., El Nadi, M.M., 1982. Sex hormones during the laying cycle of the hen. Egyp. J. Anim. Product. 22, 183-190. Kang, C.W., Nam, K.T., Olson, O.E., Carlson, C.W., 1996. Relationships between eggshell quality and biochemical parameters of calcium metabolism. Asian Aust. J. Anim. Sci. 9, 715-722. Kansal, M.L., Gangwar, P.C., 1984. Activity and distribution of carbonic anhydrase in the oviduct of the laying hen (Gallus domesticus) during egg formation at three different periods in spring and summer seasons. Acta Physiol. Pharmacol. Bulg. 10, 57-62. Kawashima, M., Kamiyoshi, M., Tanaka, K., 1982. Changes in cytoplasmic progesterone receptors and nuclear progesterone binding sites in the hen uterus (shell gland) from ovulation to oviposition. Jpn. J. Zootech. Sci. 53, 707-708. Kawashima, M., Sakae, A., Kamiyoshi, M., Tanaka, K., 1985. Changes in oestrogen receptor binding in the hen oviduct uterus (shell gland) during the period from ovulation to oviposition. Jpn. J. Zootech. Sci. 56, 680-681. Kenny, A.D., 1976. Vitamin D metabolism: physiological regulation in egg-laying Japanese quail. Am. J. Physiol. 230, 1609-1615. Khalafalla, M.K., Bessei, W., Schwarzenberg, A., 1998. Effect of mineral salts in drinking water on the domestic chicken performance - a literature review. Arch. Geflugelk 62, 26-32. Khanal, R.C., Nemere, I., 2008. Regulation of intestinal calcium transport. Annu. Rev. Nutr .28, 179-196. 56 1898 1899 1900 1901 1902 1903 1904 1905 1906 1907 1908 1909 1910 Kobayashi, S., Okamoto, S., Matsuo, T., 1981. Types of oviposition rhythm classified by length of oviposition intervals, and characteristics of each type in the quail (Coturnix coturnix japonica). Jpn. J. Zootech. Sci. 52, 850-855. 1911 1912 1913 1914 1915 1916 1917 1918 1919 1920 1921 1922 1923 1924 1925 1926 1927 1928 1929 1930 1931 1932 1933 1934 1935 1936 1937 1938 1939 1940 1941 1942 1943 1944 1945 1946 1947 1948 1949 Laklia, J., 1981. Calcium-45 transport in the distal segments of the avian oviduct an in-vitro study. Acta Vet. Acad. Sci. Hung. 29, 283-292. Kong, J., Zhang, Z., Musch, M.W., Ning, G., Sun, J., Hart, J., Bissonnette, M., Li, Y.C., 2008. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest Liver Physiol 294, G208-216. Koster, H.P.G., Hartog, A., Vanos, C.H., Bindels, R.J.M., 1995. Calbindin-D-28K facilitates cytosolic calcium diffusion without interfering with calcium signaling. Cell Calcium 18, 187-196. Kutuzova, G.D., Deluca, H.F., 2004. Gene expression profiles in rat intestine identify pathways for 1,25dihydroxyvitamin D3 stimulated calcium absorption and clarify its immunomodulatory properties. Arch. Biochem. Biophys. 432, 152-166. Lague, P.C., Tienhoven, A.v., Cunningham, F.J., 1975. Concentrations of estrogens, progesterone and LH during the ovulatory cycle of the laying chicken (Gallus domesticus). Biol. Reprod. 590-598. Acta Lambers, T.T., Bindels, R.J., Hoenderop, J.G., 2006a. Coordinated control of renal Ca 2+ handling. Kidney Int. 69, 650-654. Lambers, T.T., Mahieu, F., Oancea, E., Hoofd, L., de Lange, F., Mensenkamp, A.R., Voets, T., Nilius, B., Clapham, D.E., Hoenderop, J.G., Bindels, R.J., 2006b. Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport. Embo. J. 25, 2978-2988. Larsson, B., Nemere, I., 2003. Effect of growth and maturation on membrane-initiated actions of 1,25dihydroxyvitamin D3-II: calcium transport, receptor kinetics, and signal transduction in intestine of female chickens. J. Cell. Biochem. 90, 901-913. Lavelin, I., Meiri, N., Einat, M., Genina, O., Pines, M., 2002. Mechanical strain regulation of the chicken glypican-4 gene expression in the avian eggshell gland. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R853-R861. Lavelin, I., Meiri, N., Genina, O., Alexiev, R., Pines, M., 2001. Na +-K+-ATPase gene expression in the avian eggshell gland: distinct regulation in different cell types. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1169-R1176. Lavelin, I., Meiri, N., Pines, M., 2000. New insight in eggshell formation. Poult. Sci. 79, 1014-1017. Lavelin, I., Yarden, N., BenBassat, S., Bar, A., Pines, M., 1998. Regulation of osteopontin gene expression during egg shell formation in the laying hen by mechanical strain. Matrix Biol.. 17, 615-623. Lawson, D.E.M., 1978. Biochemical responses of the intestine to vitamin D. In: Lawson, D.E.M. (Ed.) Vitamin D, Academic Press, London, pp. 167-200 Lengemann, F.W., 1963. Over-all aspects of calcium and strontium absorption. In: Wasserman, R.H. (Ed.) The transfer of calcium and strontium acros biological membranes, Academic Press, New York, pp. 85-96 Lengemann, F.W., Comar, C.L., R.H., W., 1957. Absorption of calcium and srontium from milk and nonmilk diets. J. Nutr. 61, 571-583. Lichovnikova, M., 2007. The effect of dietary calcium source, concentration and particle size on calcium retention, eggshell quality and overall calcium requirement in laying hens. Br. Poult. Sci. 48, 71-75. Lippiello, L., Wasserman, R.H., 1975. Fluorescent antibody localization of the vitamin D-dependent calciumbinding protein in the oviduct of the laying hen. J. Histochem. Cytochem. 23, 111-116. Lomri, A., Baron, R., 1992. 1 alpha 25-dihydroxyvitamin-D3 regulates the transcription of carbonic anhydrase-II messenger RNA in avian myelomonocytes. Proc. Natl. Acad. Sci. USA 89, 4688-4692. Lundholm, C.E., 1990. The eggshell thinning action of acetazolamide: relation to the binding of Ca 2+ and carbonic anhydrase activity of shell gland homogenate. Comp. Biochem, Physiol. 95 C, 85-89. Lundholm, C.E., 1991. Influence of chlorinated hydrocarbons, HG 2+ and methyl-Hg+ on steroid hormone receptors from eggshell gland mucosa of domestic fowls and ducks. Arch. Toxicol.65, 220-227. Lundholm, C.E., 1997. DDE-induced eggshell thinning in birds: effects of p,p'-DDE on the calcium and prostaglandin metabolism of the eggshell gland. Comp. Biochem. Physiol. C 118, 113-128. Lytton, J., 2007. Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem. J. 406, 365-382. 57 1950 1951 1952 1953 1954 1955 1956 1957 1958 1959 1960 1961 1962 1963 1964 1965 1966 1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 Mann, K., Olsen, J.V., Macek, B., Gnad, F., Mann, M., 2007. Phosphoproteins of the chicken eggshell calcified layer. Proteomics 7, 106-115. Marcus, C.S., Lengemann, F.W., 1962. Use of radioyttrium to study food movement in the small intestine of the rat. J. Nutr 76, 179-182. McCormick, C.C., 2002. Passive diffusion does not play a major role in the absorption of dietary calcium in normal adults. J. Nutr. 132, 3428-3430. Melancon, M.J., Jr., DeLuca, H.F., 1970. Vitamin D stimulation of calcium-dependent adenosine triphosphatase in chick intestinal brush borders. Biochemistry 9, 1658-1664. Migicovsky, B.B., Nielson, A.M., 1951. Calcium absorption and vitamin D in the chick. Arch. Biochem. 34, 105-111. Miller, S.C., 1992. Calcium homeostasis and mineral turnover in the laying hen. In: Whithead, C.C. (Ed.) Bone Biology and Skeletal Disorders in Poultry, vol. 23. Carfax, Abingdon, pp. 103-116 Minghetti, P.P., Cancela, L., Fujisawa, Y., Theofan, G., Norman, A.W., 1988. Molecular structure of the chicken vitamin D-induced calbindin-D28K gene reveals eleven exons, six Ca2+-binding domains, and numerous promoter regulatory elements. Mol. Endocrinol. 2, 355-367. Minvielle, F., Monvoisin, J.L., Costa, J., Maeda, Y., 2000. Long-term egg production and heterosis in quail lines after within-line or reciprocal recurrent selection for high early egg production. Br. Poult. Sci. 41, 150-157. Mongin, P., 1976a. Composition of crop and gizzard contents in the laying hen. Br. Poult. Sci. 17, 499-507. Mongin, P., 1976b. Ionic constituents and osmolality of the small intestinal fluids of the laying hen. Br. Poult. Sci.. 17, 383-392. Mongin, P., 1978. Studies on the avian shell gland during egg formation: the effect of acetazolamide on the composition of the mucosa. Br. Poult. Sci. 19, 501-509. Mongin, P., Lacassagne, L., 1964. [Physiology of the Formation of the Chicken Egg Shell and Acid-Base Equilibrium of the Blood.]. C. R. Hebd. Seances Acad. Sci. 258, 3093-3094. Mongin, P., Sauveur, B., 1970. [Composition of the uterine fluid and albumin during the stay of ovum in the uterus in domestic hens]. C R Hebd Seances Acad Sci. D 270, 1715-1718. Monroe, D.G., Berger, R.R., Sanders, M.M., 2002. Tissue-protective effects of estrogen involve regulation of caspase gene expression. Mol. Endocrinol. 16, 1322-1331. Montecuccoli, G., Bar, A., Risenfeld, G., Hurwitz, S., 1977a. The response of 25-hydroxycholecalciferol-1hydroxylase activity, intestinal calcium absorption, and calcium-binding protein to phosphate deficiency in chicks. Comp. Biochem, Physiol. 57A, 331-334. Montecuccoli, G., Hurwitz, S., Cohen, A., Bar, A., 1977b. The role of 25-hydroxycholecalciferol-1-hydroxylase in the responses of calcium absorption to the reproductive activity in birds. Comp. Biochem. Physiol. 57A, 335-339. Morrissey, R.L., Wasserman, R.H., 1971. Calcium absorption and calcium-binding protein in chicks on differing calcium and phosphorus intakes. Am. J. Physiol. 220, 1509-1515. Musser, M.A., Bacon, W.L., Nestor, K.E., 1977. Calcium binding protein in the turkey and in the Japanese quail. Poult. Sci. 56, 1657-1658. Nakada, T., 1990. Presence of egg in the oviduct uterus stimulates shell gland calcium secretion in the hen. Jap. Poult. Sci. 27, 162-163. Nakada, T., Koga, O., 1990. Stimulation of secretion of shell gland fluid and calcium by the presence of ovum or ovum-like mass containing artificial yolk in the oviduct uterus of the hen. Jap. Poult. Sci. 27, 2128. Nakamura, I., Rodan, G.A., Duong, l.T., 2003. Regulatory mechanism of osteoclast activation. J. Electron. Microsc. 52, 527-533. Navickis, R.J., Dial, O.K., Katzenellenbogen, B.S., Nalbandov, A.V., 1979a. Effects of gonadal hormones on calcium-binding protein in chick duodenum. Am. J. Physiol. 237, E408-E417. Navickis, R.J., Katzenellenbogen, B.S., Nalbandov, A.V., 1979b. Effects of the sex steroid hormones and vitamin D3 on calcium-binding proteins in the chick shell gland. Biol. Reprod. 21, 1153-1162. Nellans, H.N., Kimberg, D.V., 1978. Cellular and paracellular calcium transport in rat ileum: effects of dietary calcium. Am. J. Physiol. 235, E726-E737. 58 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 2038 2039 2040 2041 2042 2043 2044 2045 2046 2047 2048 2049 2050 2051 2052 2053 2054 Nemere, I., Larsson, D., 2002. Does PTH have a direct effect on intestine? J. Cell. Biochem. 86, 29-34. Nemere, I., Norman, A.W., 1990. Transcaltachia, vesicular calcium transport, and microtubule-associated calbindin-D28K: emerging views of 1,25-dihydroxyvitamin D3-mediated intestinal calcium absorption. Miner. Electrolyte Metab. 16, 109-114. Nicolaysen, R., 1943. The absorption of calcium as a function of the body starvation with calcium. Acta Physiol. Scand. 5, 200-211. Nicolaysen, R., Eeg-Larsen, N., Malm, O.J., 1953. Physiology of calcium metabolism. Physiol. Rev. 33, 424444. Niemeyer, B.A., 2005. Structure-function analysis of TRPV channels. Naunyn Schmiedebergs Arch. Pharmacol. 371, 285-294. Nijenhuis, T., Hoenderop, J.G., Bindels, R.J., 2005. TRPV5 and TRPV6 in Ca 2+ (re)absorption: regulating Ca2+ entry at the gate. Pflugers Arch. 451, 181-192. Noda, M., Vogel, R.L., Craig, A.M., Prahl, J., Deluca, H.F., Denhardt, D.T., 1990. Identification of a DNA sequence responsible for binding of the 1,25-Dihydroxyvitamin-D3 receptor and 1,25dihydroxyvitamin-D3 enhancement of mouse secreted phosphoprotein-1 (SPP-1 or osteopontin) gene expression. Proc. Natl. Acad. Sci. USA 87, 9995-9999. Norman, A.W., 1995. The vitamin D endocrine system: manipulation of structure-function relationships to provide opportunities for development of new cancer chemopreventive and immunosuppressive agents. J. Cell. Biochem. 218-225. Norman, A.W., 2006. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 147, 5542-5548. Norman, A.W., Okamura, W.H., Bishop, J.E., Henry, H.L., 2002. Update on biological actions of 125(OH)2vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D3. Mol. Cell. Endocrinol. 197, 1-13. Nys, Y., 1987. Progesterone and testosterone elicit increases in the duration of eggshell formation in domestic hens. Br. Poult. Sci.. 28, 57-68. Nys, Y., Baker, K., Bouillon, R., Vanbaelen, H., Lawson, D.E.M., 1992a. Regulation of calbindin-D-28K and is messenger RNA in the intestine of the domestic hen. Gen. Comp. Endocrinol. 86, 460-468. Nys, Y., Baker, K., Lawson, D.E., 1992b. Estrogen and a calcium flux dependent factor modulate the calbindin gene expression in the uterus of laying hens. Gen. Comp. Endocrinol. 87, 87-94. Nys, Y., de Laage, X., 1984a. Effects of suppression of eggshell calcification and of 1,25(OH) 2D3 on Mg2+, Ca2+ and Mg2+HCO-3 ATPase, alkaline phosphatase, carbonic anhydrase and CaBP levels--I. The laying hen uterus. Comp. Biochem, Physiol. A 78, 833-838. Nys, Y., de Laage, X., 1984b. Effects of suppression of eggshell calcification and of 1,25(OH) 2D3 on Mg2+, Ca2+ and Mg2+HCO-3 ATPase, alkaline phosphatase, carbonic anhydrase and CaBP levels--II. The laying hen intestine. Comp. Biochem, Physiol. A 78, 839-844. Nys, Y., Gautron, J., McKee, M.D., Garcia Ruiz, J.M., Hincke, M.T., 2001. Biochemical and functional characterization of egg shell matrix proteins in hens. World's Poult. Sci. J. 57, 401-413. Nys, Y., Hincke, M.T., Arias, J.L., Garcia Ruiz, J.M., Solomon, S.E., 1999. Avian eggshell mineralization. Poult. Avian Biol. Rev. 10, 143-166. Nys, Y., Mayel Afshar, S., Bouillon, R., Van Baelen, H., Lawson, D.E., 1989. Increases in calbindin D 28K mRNA in the uterus of the domestic fowl induced by sexual maturity and shell formation. Gen. Comp. Endocrinol. 76, 322-329. Nys, Y., Mongin, P., 1980. Jejunal calcium permeability in laying hens during egg formation. Reprod. Nutr. Dev. 20, 155-161. Nys, Y., N'Guyen, T.M., Garabedian, M., 1984c. Involvement of 1,25-dihydroxycholecalciferol in the short- and long-term increase of intestinal calcium absorption in laying hens: stimulation by gonadal hormones is partly independent of 1,25-dihydroxycholecalciferol. Gen. Comp. Endocrinol. 54, 59-68. Nys, Y., N'Guyen, T.M., Williams, J., Etches, R.J., 1986a. Blood levels of ionized calcium, inorganic phosphorus, 1,25-dihydroxycholecalciferol and gonadal hormones in hens laying hard-shelled or shell-less eggs. J. Endocrinol. 111, 151-157. Nys, Y., Parkes, C.O., Thomasset, M., 1986b. Effects of suppression and resumption of shell formation and parathyroid hormone on uterine calcium-binding protein, carbonic anhydrase activity, and intestinal calcium absorption in hens. Gen. Comp. Endocrinol. 64, 293-299. 59 2055 2056 2057 2058 2059 2060 2061 2062 2063 2064 2065 2066 2067 2068 2069 2070 2071 2072 2073 2074 2075 2076 2077 2078 2079 2080 2081 2082 2083 2084 2085 2086 2087 2088 2089 2090 2091 2092 2093 2094 2095 2096 2097 2098 2099 2100 2101 2102 2103 2104 2105 2106 Nys, Y., Zawadzki, J., Gautron, J., Milles, A.D., 1991. Whitening of brown-shelled eggs: mineral composition of uterine fluid and rate of protoporphyrin deposition. Poult. Sci. 70, 1236-1245. Ohashi, T., Hirakata, Y., Hetsuka, K., Yamauchi, K., Haga, S., 1984. Relationships of the egg production rate and egg shell quality with uterus carbonic anhydrase activity in laying hens. Bulletin of the Faculty of Agriculture, Miyazaki University 31, 91-95. Oka, T., Schimke, R.T., 1969. Interaction of estrogen and progesterone in chick oviduct development. I. Antagonistic effect of progesterone on estrogen-induced proliferation and differentiation of tubular gland cells. J. Cell. Biol. 41, 816-831. Panheleux, M., Kalin, O., Gautron, J., Nys, Y., 1999. Features of eggshell formation in guinea fowl: kinetics of shell deposition, uterine protein secretion and uterine histology. Br. Poult. Sci.. 40, 632-643. Pansu, D., Bellaton, C., Roche, C., Bronner, F., 1983. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am. J. Physiol. 244, G695-G700. Pearson, T.W., Goldner, A.M., 1973. Calcium transport across avian uterus. I. Effects of electrolyte substitution. Am. J. Physiol. 225, 1508-1512. Pearson, T.W., Goldner, A.M., 1974. Calcium transport across avian uterus. II. Effects of inhibitors and nitrogen. Am. J. Physiol. 227, 465-468. Pearson, T.W., Pryor, T.J., Goldner, A.M., 1977. Calcium transport across avian uterus. III. Comparison of laying and nonlaying birds. Am. J. Physiol. 232, E437-E443. Perez, A.V., Picotto, G., Carpentieri, A.R., Rivoira, M.A., Peralta Lopez, M.E., Tolosa de Talamoni, N.G., 2008. Minireview on regulation of intestinal calcium absorption. Emphasis on molecular mechanisms of transcellular pathway. Digestion 77, 22-34. Pike, J.W., Shevde, N.K., 2005. The vitamin D receptor. In: Feldman, D., Pike, W.J., Glorieux, F.H. (Eds.), Vitamin D, Elsevier Academic Press, Burlington, MA; San Diego, CA; London, UK, pp. 167-192. Pines, M., Knopov, V., Bar, A., 1995. Involvement of osteopontin in egg shell formation in the laying chicken. Matrix Biol. 14, 765-771. Potts, J.T., 2005. Parathyroid hormone: past and present. J. Endocrinol. 187, 311-325. Prince, C.W., Butler, W.T., 1987. 1,25-Dihydroxyvitamin D3 regulates the biosynthesis of osteopontin, a bonederived cell attachment protein, in clonal osteoblast-like osteosarcoma cells. Coll. Relat. Res. 7, 305313. Purkerson, J.M., Schwartz, G.J., 2007. The role of carbonic anhydrases in renal physiology. Kidney Int. 71, 103115. Qin, X., Klandorf, H., 1993. Effect of estrogen in relation to dietary vitamin D3 and calcium on activity of intestinal alkaline phosphatase and Ca-ATPase in immature chicks. Gen. Comp. Endocrinol. 90, 318327. Quelo, I., Kahlen, J.P., Rascle, A., Jurdic, P., Carlberg, C., 1994. Identification and characterization of a vitamin D-3 response element of chicken carbonic anhydrase-II. DNA Cell Biol. 13, 1181-1187. Rabon, H.W., Jr., Roland, D.A., Sr., Clark, A.J., 1991. Uterine calcium-binding protein activity of nonlaying hens and hens laying hard-shelled or shell-less eggs. Poult. Sci. 70, 2280-2283. Radwan, A.A., Gado, M.S., El Habbak, M.M.E., 1985. Study on active transport of calcium in laying hens. Egyp. J. Anim. Product. 25, 233-247. Ramasamy, I., 2006. Recent advances in physiological calcium homeostasis. Clin. Chem. Lab. Med. 44, 237273. Raval-Pandya, M., Porta, A.R., Christakos, S., 1999. Mechanism of action of 1,25-dihydroxyvitamin D3 on intestinal calcium absorption and renal calcium. In: Holick, M.F. (Ed.) Vitamin D. Physiology, Molecular Biology and Clinical Applications, Humana Press, Totowa, New Jersey, pp. 163-194 Rieser, J.W., Smith, J.D., Burke, W.H., 1972. Composition of turkey uterine fluid. Poult. Sci. 51, 203-206. Rittling, S.R., Denhardt, D.T., 1999. Osteopontin function in pathology: lessons from osteopontin-deficient mice. Exp. Nephrol. 7, 103-113. Rosenberg, J., Hurwitz, S., Bar, A., 1986. Regulation of kidney calcium-binding protein in the bird (Gallus domesticus). Comp. Biochem, Physiol. A 83, 277-281. Rubin, J., Rubin, C., Jacobs, C.R., 2006. Molecular pathways mediating mechanical signaling in bone. Gene 367, 1-16. 60 2107 2108 2109 2110 2111 2112 2113 2114 2115 2116 2117 2118 2119 2120 2121 2122 2123 2124 2125 2126 2127 2128 2129 2130 2131 2132 2133 2134 2135 2136 2137 2138 2139 2140 2141 2142 2143 2144 2145 2146 2147 2148 2149 2150 2151 2152 2153 2154 2155 2156 Salevsky, E., Jr., Leach, R.N., Jr., 1980. Studies on the organic components of shell gland fluid and the hen's egg shell. Poult. Sci. 59, 438-443. Sallis, J.D., Holdsworth, E.S., 1962. Influence of vitamin D on calcium absorption in the chick. Am. J. Physiol. 203, 497-505. Sasayama, Y., 1999. Hormonal control of Ca homeostasis in lower vertebrates: considering the evolution. Zool. Sci. 16, 857-869. Sauveur, B., Mongin, P., 1978. Studies on the avian shell gland during egg formation: the effect of acetazolamide on the transfer of ions to the albumen. Br. Poult. Sci.. 19, 511-520. Schachter, D., Rosen, S.M., 1959. Active transport of 45Ca by the small intestine and its dependence on vitamin D. Am. J. Physiol. 196, 357-362. Scheideler, S.E., 1998. Eggshell calcium effects on egg quality and ca digestibility in first- or third-cycle laying hens. J. Appl. Poult. Res. 1, 69-74. Schneeberger, E.E., Lynch, R.D., 2004. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286, C1213-1228. Schoeber, J.P., Hoenderop, J.G., Bindels, R.J., 2007. Concerted action of associated proteins in the regulation of TRPV5 and TRPV6. Biochem. Soc. Trans. 35, 115-119. Scott, T.A., Balnave, D., 1991. Influence of temperature, dietary energy, nutrient concentration and selfselection feeding on the retention of dietary energy, protein and calcium by sexually-maturing egglaying pullets. Brit. Poult. Sci. 32, 1005-1016. Shahabi, N.A., Norton, H.W., Nalbandov, A.V., 1975. Steroid levels in follicles and the plasma of hens during the ovulatory cycle. Endocrinology 96, 962-968. Sharp, P.J., 2005. Photoperiodic regulation of seasonal breeding in birds. Ann N Y Acad Sci 1040, 189-199. Shepherd, N., Graham, V., Trevedi, B., Creazzo, T.L., 2007. Changes in regulation of sodium/calcium exchanger of avian ventricular heart cells during embryonic development. Am. J. Physiol. Cell Physiol. 292, C1942-1950. Shimada, K., Etches, R.J., 1986. Uterine contractile activities of chicken, quail and turkey during laying cycles. Jap. Poult. Sci. 23, 125-130. Silverman, S.L., 2003. Calcitonin. Endocrinol. Metab. Clin. North. Am. 32, 273-284. Singh, R., Joyner, C.J., Peddie, M.J., Taylor, T.G., 1986. Changes in the concentrations of parathyroid hormone and ionic calcium in the plasma of laying hens during the egg cycle in relation to dietary deficiencies of calcium and vitamin D. Gen. Comp. Endocrinol. 61, 20-28. Soares, J.H., Kerr, J.M., Gray, R.W., 1995. 25-hydroxycholecalciferol in poultry nutrition. Poult. Sci. 74, 19191934. Sodek, J., Ganss, B., McKee, M.D., 2000. Osteopontin. Crit. Rev. Oral. Biol. Med. 11, 279-303. Soledad Fernandez, M., Moya, A., Lopez, L., Arias, J.L., 2001. Secretion pattern, ultrastructural localization and function of extracellular matrix molecules involved in eggshell formation. Matrix Biol. 19, 793-803. Solomon, S.E., 1991. Egg & Eggshell Quality. Volfe Publishing Ltd, London. Sonoda, Y., Kai, O., Imai, K., 1997. Egg laying and ovarian follicular growth in Japanese quail under continuous lighting. Jap. Poul. Sci. 34, 308-317. Stains, J.P., Weber, J.A., Gay, C.V., 2002. Expression of Na +/Ca2+ exchanger isoforms (NCX1 and NCX3) and plasma membrane Ca2+ ATPase during osteoblast differentiation. J. Cell. Biochem. 84, 625-635. Stokes, D.L., Green, N.M., 2003. Structure and function of the calcium pump. Annu. Rev. Biophys. Biomol. Struct. 32, 445-468. Striem, S., 1990. Regulation of calcium-binding protein (calbindin 28KDA) synthesis in the avian uterus. Master Sci. degree, Hebrew university, Jerusalem, Israel. Striem, S., Bar, A., 1989. Vitamin D metabolism and expression in estrogen-treated female birds. Abstracts; The XXI European Symposium on Calcified Tissues. Calcif. Tiss. Int. 44, s-26. Striem, S., Bar, A., 1991. Modulation of quail intestinal and egg shell gland calbindin (M r 28,000) gene expression by vitamin D3, 1,25-dihydroxyvitamin D3 and egg laying. Mol. Cell. Endocrinol. 75, 169177. 61 2157 2158 2159 2160 2161 2162 2163 2164 2165 2166 2167 2168 2169 2170 2171 2172 2173 2174 2175 2176 2177 2178 2179 2180 2181 2182 2183 2184 2185 2186 2187 2188 2189 2190 2191 2192 2193 2194 2195 2196 2197 2198 2199 2200 2201 2202 2203 2204 2205 2206 2207 2208 2209 Strittmatter, C.F., 1972. Phosphatase activities of chicken liver and duodenum: characteristics, levels during development, and hydrocortisone-induced changes. Biochim. Biophys. Acta 284, 183-195. Sugiyama, T., Kikuchi, H., Hiyama, S., Nishizawa, K., Kusuhara, S., 2007. Expression and localisation of calbindin D28k in all intestinal segments of the laying hen. Br. Poult. Sci.. 48, 233-238. Sunahra, T., Annaka, A., Watanabe, E., Ishibashi, T., 1989. Apparent absorption site of calcium and phosphorus determined by chromic oxide and polyethylene glycol as markers in the laying hen. Bulletin of the Faculty of Agriculture, Niigata University 41, 29-36. Talmage, R.V., Mobley, H.T., 2008. Calcium homeostasis: reassessment of the actions of parathyroid hormone. Gen Comp Endocrinol 156, 1-8. Tanaka, K., 1980. Hormone-receptor interaction II: Steroid hormones. In: Epple, E., Stetson, M.H. (Eds.), Avian Endocrinology, Academic Press, New York, pp. 17-31 Tang, V.W., Goodenough, D.A., 2003. Paracellular ion channel at the tight junction. Biophys. J. 84, 1660-1673. Taylor, A.N., Wasserman, R.H., 1967. Vitamin D 3-induced calcium-binding protein: partial purification, electrophoretic visualization, and tissue distribution. Arch. Biochem. Biophys. 119, 536-540. Taylor, A.N., Wasserman, R.H., 1969. Correlations between the vitamin D-induced calcium binding protein and intestinal absorption of calcium. Fed. Proc. 28, 1834-1838. Taylor, A.N., Wasserman, R.H., 1972. Vitamin D-induced calcium binding protein: comparative aspects in kidney and intestine. Am J Physiol. 223, 110-114. Taylor, T.G., Kirkley, J., 1967. The absorption and excretion of minerals by laying hens in relation to egg shell formation. Br. Poult. Sci. 8, 289-295. Thiede, M.A., Harm, S.C., Mckee, R.L., Grasser, W.A., Duong, L.T., Leach, R.M., 1991. Expression of the parathyroid hormone-related protein gene in the avian oviduct - potential role as a local modulator of vascular smooth muscle tension and shell gland motility during the egg-laying cycle. Endocrinology 129, 1958-1966. Thomasset, M., 1997. Calbindin-D9k. In: Feldman, D., Glorieux, Pike (Eds.), Vitamin D, Academic Press, pp. 223-232 Turner, R.T., Eliel, L.P., 1978. Nuclear estrogen receptor in the reproductive tract of laying Japanese quail. Gen. Comp. Endocrin. 34, 141-148. Ussing, H.H., 1949. Transport of ions across cellular membranes. Physiol. Rev. 29, 127-155. Ussing, H.H., Zerahn, K., 1951. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol. Scand. 23, 110-127. van Abel, M., Hoenderop, J.G., Bindels, R.J., 2005. The epithelial calcium channels TRPV5 and TRPV6: regulation and implications for disease. Naunyn Schmiedebergs Arch. Pharmacol. 371, 295-306. Van Cromphaut, S.J., Rummens, K., Stockmans, I., Van Herck, E., Dijcks, F.A., Ederveen, A.G., Carmeliet, P., Verhaeghe, J., Bouillon, R., Carmeliet, G., 2003. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J. Bone. Miner. Res. 18, 1725-1736. van de Graaf, S.F., Bindels, R.J., Hoenderop, J.G., 2007. Physiology of epithelial Ca 2+ and Mg2+ transport. Rev. Physiol. Biochem. Pharmacol. 158, 77-160. van de Velde, J.P., Loveridge, N., Vermeiden, J.P.W., 1984. Parathyroid hormone responses to calcium stress during eggshell calcification. Endocrinology 115, 1901-1904. van der Eerden, B.C., Hoenderop, J.G., de Vries, T.J., Schoenmaker, T., Buurman, C.J., Uitterlinden, A.G., Pols, H.A., Bindels, R.J., van Leeuwen, J.P., 2005. The epithelial Ca 2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc. Natl. Acad. Sci. U S A 102, 17507-17512. Van Itallie, C.M., Anderson, J.M., 2006. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68, 403-429. Van-Abel, M., Hoenderop, J.G.J., van-der-Kemp, A., Van-Leeuwen, J.P.T.M., Bindels, R.J.M., 2003. Regulation of the epithelial Ca2+ channels in small intestine as studied by quantitative mRNA detection. Am. J. Physiol. 285, G78-G85. Vanmontfort, D., Berghman, L.R., Rombauts, L., Verhoeven, G., Decuypere, E., 1994. Changes of immunoreactive inhibin, follicle-stimulating hormone, luteinizing hormone, and progesterone in plasma after short-term food deprivation and during the ovulatory cycle of the domestic hen. Gen. .Comp. Endocrin. 95, 117-124. 62 2210 2211 2212 2213 2214 2215 2216 2217 2218 2219 2220 2221 2222 2223 2224 2225 2226 2227 2228 2229 2230 2231 2232 2233 2234 2235 2236 2237 2238 2239 2240 2241 2242 2243 2244 2245 2246 2247 2248 2249 2250 2251 2252 2253 2254 2255 2256 2257 2258 2259 2260 2261 Venkatachalam, K., Montell, C., 2007. TRP channels. Annu. Rev. Biochem. 76, 387-417. Vetter, A.E., O' Grady, S.M., 2005. Sodium and anion transport across the avian uterine (shell gland) epithelium. J. Exp. Biol. 208, 479-486. Waddington, D., Peddie, J., Dewar, W.A., Gilbert, A.B., 1989. Regulation of net intestinal calcium uptake in hens laying obligatory soft-shelled eggs. Br. Poult. Sci.. 30, 341-351. Walzem, R.L., 1996. Lipoproteins and the laying hen: form follows function. Poult. Avian Biol. Rev. 7, 31-64. Walzem, R.L., Hansen, R.J., Williams, D.L., Hamilton, R.L., 1999. Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 129, 467S-472S. Wasserman, R.H., 1962. Studies on vitamin D 3 and the intestinal absorption of calcium and other ions in the rachitic chick. J. Nutr. 77, 69-80. Wasserman, R.H., 2004. Vitamin D and the dual processes of intestinal calcium absorption. J. Nutr. 134, 31373139. Wasserman, R.H., 2005. Vitamin D and the intestinal absorption of calcium: A vew and overview. In: Feldman, D., Pike, W.J., Glorieux, F.H. (Eds.), Vitamin D, Elsevier Academic Press, Burlington, MA; San Diego, CA; London, UK, pp. 411-428. Wasserman, R.H., Combs, G.F., Jr., 1978. Relation of vitamin D-dependent intestinal calcium-binding protein to calcium absorption during the ovulatory cycle in Japanese quail. Proc. Soc. Exp. Biol. Med. 159, 286287. Wasserman, R.H., Smith, C.A., Brindak, M.E., De Talamoni, N., Fullmer, C.S., Penniston, J.T., Kumar, R., 1992. Vitamin D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology 102, 886-894. Wasserman, R.H., Smith, C.A., Smith, C.M., Brindak, M.E., Fullmer, C.S., Krook, L., Penniston, J.T., Kumar, R., 1991. Immunohistochemical localization of a calcium pump and calbindin-D28k in the oviduct of the laying hen. Histochemistry 96, 413-418. Wasserman, R.H., Taylor, A.N., 1966. Vitamin D-induced calcium-binding protein in chick intestinal mucosa. Science (Washington, DC) 152, 791-793. Wasserman, R.H., Taylor, A.N., 1968. Vitamin D-dependent calcium-binding protein. Response to some physiological and nutritional variables. J. Biol. Chem. 243, 3987-3993. Watanabe, E., Annaka, A., Ishibahi, T., 1992. Fluctuations of Fluctuations of plasma calcium and inorganic phosphorus concentrations in the uterine duodenal and femoral veins and common carotid artery during the egg formation cycle in laying hens. Jap. Poult. Sci. 29, 115-126. Weber, K., Erben, R.G., Rump, A., Adamski, J., 2001. Gene structure and regulation of the murine epithelial calcium channels ECaC1 and 2. Biochem. Biophys. Res. Commun. 289, 1287-1294. Whitehead, C.C., 1998. A review of nutritional and metabolic factors involved in dyschondroplasia in poultry. J. Appl. Anim. Res. 13, 1-16. Wideman, R.F., Jr., Buss, E.G., 1985. Arterial blood gas, pH, and bicarbonate values in laying hens selected for thick or thin eggshell production. Poult. Sci. 64, 1015-1019. Wilson, P.W., Harding, M., Lawson, D.E.M., 1985. Putative amino acid sequence of chick calcium-binding protein deduced from complementary DNA sequence. Nucleic Acids Res. 13, 8867-8881. Wilson, P.W., Rogers, J., Harding, M., Pohl, V., Pattyn, G., Lawson, D.E., 1988. Structure of chick chromosomal genes for calbindin and calretinin. J. Mol. Biol. 200, 615-625. Wu, J.C.Y., Smith, M.W., Mitchell, M.A., Peacock, M.A., Turvey, A., Keable, S.J., 1993. Enterocyte expression of calbindin, calbindin mRNA and calcium transport increases in jejunal tissue during onset of egg production in the fowl (Gallus domesticus). Comp. Biochem, Physiol. [A] 106, 263-269. Wu, J.C.Y., Smith, M.W., Turvey, A., Keable, S.J., Colston, K.W., 1994. Differential regulation of vitamin d receptor and intestinal calcium transport occurring during sexual maturation in the fowl (Gallus domesticus). Comp. Biochem, Physiol. A 109, 713-720. Yamamoto, T., Ozawa, H., Nagai, H., 1985. Histochemical studies of Ca-ATPase, succinate and NAD+dependent isocitrate dehydrogenases in the shell gland of laying Japanese quails: with special reference to calcium-transporting cells. Histochemistry 83, 221-226. Yosefi, S., Braw-Tal, R., Bar, A., 2003. Intestinal and eggshell calbindin and bone ash as influenced by age of the laying hen and molting. Comp. Biochem, Physiol. A 136, 673-682. 63 2262 2263 2264 2265 2266 2267 2268 2269 2270 2271 2272 2273 2274 Yoselewitz, I., Balnave, D., 1989. The influence of saline drinking water on the activity of carbonic anhydrase in the shell gland of laying hens. Aust. J. Agric. Res. 40, 1111-1115. Yoshimura, Y., Ohira, H., Tamura, T., 1997. Immunocytochemical localization of vitamin D receptors in the shell gland of immature, laying, and molting hens. Gen. Comp. Endocrinol. 108, 282-289. Zelinski, J.M., Sykes, D.E., Weiser, M.M., 1991. The effect of vitamin D on rat intestinal plasma membrane CA-pump mRNA. Biochem. Biophys. Res. Commun. 179, 749-755. Zhu, Y., Goff, J.P., Reinhardt, T.A., Horst, R.L., 1998. Pregnancy and lactation increase vitamin D-dependent intestinal membrane calcium adenosine triphosphatase and calcium binding protein messenger ribonucleic acid expression. Endocrinology 139, 3520-3524.