CHAP Seven/Eight Outline: Wade Alkene Synthesis and Reactions
advertisement

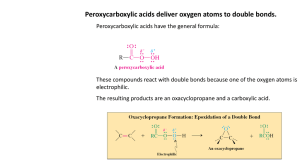
CHAP SEVEN/EIGHT OUTLINE: WADE ALKENE SYNTHESIS AND REACTIONS 1) Structure and Properties a) The pi bond and hindered C-C rotation i) cis- / trans- nomenclature ii) E/Z nomenclature b) Molecular Orbital Energy Diagram of ethene c) The E/Z Nomenclature System d) Relative Stability via Heats of Hydrogenation 2) Formation of Alkenes a) From Alkyl Halides via E2 with strong bases (Ch 6 Review; Zaitsev product unless anti-coplanar transition state not possible) Section 7-9 b) From Alcohols via E1 with strong acid catalysts (rearrangement likely) Section 7-10 c) Skip 7-9D d) Skip 7-11 3) Reactions of Alkenes CHAPTER EIGHT a) Alkenes as Nucleophile i) Addition of HX in absence of peroxides to form Markovnikov Alkyl Halide (both enantiomers) Mechanism Stereochemistry Reaction Energy Profile Regio-Selectivity: Markovnikov’s Rule Carbocation stability (review) o Inductive effects o Hyperconjugation o Carbocation rearrangement ii) Addition of HOH or HOR (1) Via reaction with H2SO4 and water or alcohol to make Markovnikov Alcohol or Ether (rearrangement likely) Mech 8-4 (2) Via reaction with Hg(OAc)2 and H2O or ROH followed by NaBH4 to make Markovnikov Alcohol or Ether (no rearrangement) Mech 8-5 iii) Hydroboration-Oxidation: Addition of BH3•THF followed by H2O2, OH– to form ANTI-Markovnikov Alcohol Mech 8-6 iv) Addition of X2 to form vicinal dihalide Mech 8-7 v) Addition of Carbenes to Alkenes (Section 8-11) (1) Diazomethane decomposition to methylene carbene (2) Simmons-Smith Reaction: Addition of CH2I2, Zn(Cu) ICH2ZnI decomposition to methylene carbene (3) Haloform in KOH decomposition to dihalocarbene b) Oxidation Reactions i) Formation of Epoxides with Peroxyacids: Concerted Mechanism ii) Formation of vicinal diols (1) Anti dihydroxylation via epoxide ring opening (2) Syn dihydroxylation via reaction with OsO4 (3) Syn dihydroxylation via KMnO4 under mild conditions CHAP SEVEN/EIGHT OUTLINE: WADE ALKENE SYNTHESIS AND REACTIONS iii) Oxidative Cleavage (1) With KMnO4 under basic conditions or higher temp to form ketones/carboxylates (2) Ozonolysis With O3 followed by (CH3)2S to form ketones/aldehydes c) Free Radical Reactions i) Addition of HX in presence of peroxides to form ANTI-Markovnikov Alkyl halide (both enantiomers) Mech 8-3 ii) Addition of NBS in presence of peroxides to form allylic alkyl halide (Chap 6-6 review) iii) Polymerization Section 8-16B d) Oxidation-Reduction i) Reduction to alkane via syn addition of H2 with metal catalyst (Pd, Pt, Ni, Rh, Ru, etc) Section 8-10 ii) Oxidation to Epoxide via reaction with peroxyacid Section 8-12; Mech 8-9 iii) Syn-Dihydroxylation Section 8-14 (1) with OsO4 and H2O2 (2) with KMnO4, OH– iv) Oxidative Cleavage Section 8-15 (1) with O3 followed by Zn or (CH3)2S to aldehydes and/or ketones (2) with KMnO4 when concentrated in base or with heat or acidic solution to carboxylic acids and/or ketones v) Olefin Metathesis with Mo (Shrock) or Ru (Grubbs) catalyst Section 8-17; Mech 8-11 LEARNING OUTCOMES: Identify electrophiles and nucleophiles according to their roles in well characterized chemical reactions Understand the most common chemical reactions involving alkenes including analysis of regio- and stereo- selectivity in product formation, step-wise mechanisms and identification of intermediates, and advantages/ disadvantages of similar reactions. Design a multiple-step synthesis of a target compound utilizing alkene chemistry. Propose a mechanism for a molecular transformation by applying principles learned from known reactions. Sketch a reaction Energy profile for a reaction from its known mechanism. Understand how chirality and optical activity depend on mechanism and the stereochemistry of the starting materials. Name alkenes according to Cahn-Ingold-Prelog E/Z Nomenclature. Predict relative stability of alkenes from Heats of hydrogenation. Recognize structural features of a molecule that are key to its stability and reactivity. CHAP SEVEN/EIGHT OUTLINE: WADE ALKENE SYNTHESIS AND REACTIONS SAMPLE EXAM PROBLEMS: 1. Provide a mechanism for the following reaction. Use curved arrows and include all lone electron pairs and formal charge. 2. Draw the following molecule: 4R-(Z)-2,4-dibromo-3,4-dimethyl-2-hexene: 3. Provide the missing reagents to complete the following chemical transformation: 4. Consider the reaction below and tell why it DOES NOT provide high yields of the product shown. Draw alternative products, if any are formed